ILC3s通过宿主肠道半乳糖基化调节肠道微生物群,限制小鼠病原体感染
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
宿主免疫和共生菌协同维持肠道稳态并介导对病原体的定植抗性。然而,分子和细胞机制尚不清楚。本研究通过小鼠啮齿柠檬酸杆菌感染模型,发现第3组先天淋巴样细胞(ILC3s)可以通过调节肠道微生物群来保护宿主免受感染。在机制上,ILC3s可以通过il -22依赖性调节小鼠肠道半乳糖基化来控制肠道生态失调。ILC3缺乏导致肠道半乳糖基化增加,结肠粘液中共生嗜粘阿克曼氏菌的扩增。嗜粘单胞菌及其衍生代谢产物琥珀酸盐的增加进一步促进了病原菌毒力因子tir和ler的表达,导致对啮齿鼠的易感性增加。总之,我们的数据揭示了ILC3s通过调节肠道糖基化和肠道微生物群代谢来保护病原体感染的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

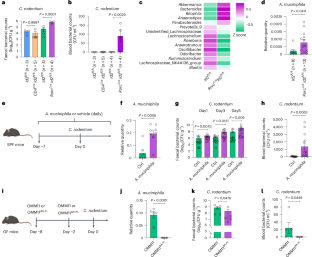
ILC3s regulate the gut microbiota via host intestinal galactosylation to limit pathogen infection in mice
Host immunity and commensal bacteria synergistically maintain intestinal homeostasis and mediate colonization resistance against pathogens. However, the molecular and cellular mechanisms remain unclear. Here, with a mouse infection model of Citrobacter rodentium, a natural mouse intestinal pathogen that mimics human enteropathogenic Escherichia coli and enterohaemorrhagic Escherichia coli, we find that group 3 innate lymphoid cells (ILC3s) can protect the host from infection by regulating gut microbiota. Mechanistically, ILC3s can control gut dysbiosis through IL-22-dependent regulation of intestinal galactosylation in mice. ILC3 deficiency led to an increase in intestinal galactosylation and the expansion of commensal Akkermansia muciniphila in colonic mucus. The increased A. muciniphila and A. muciniphila-derived metabolic product succinate further promoted the expression of pathogen virulence factors tir and ler, resulting in increased susceptibility to C. rodentium infection. Together, our data reveal a mechanism for ILC3s in protecting against pathogen infection through the regulation of intestinal glycosylation and gut microbiota metabolism. Akkermansia muciniphila can promote intestinal pathogen infection in mice via microbial metabolite succinate production, but the expansion of A. muciniphila is strictly controlled by ILC3s through the regulation of intestinal galactosylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


