不锈钢上多孔CoP涂层:作为酸性析氢反应电催化剂的应用
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发具有合理活性和稳定性的新型酸性析氢催化剂对于降低PEM电解槽的成本和促进其广泛商业化至关重要。在这项研究中,我们提出了一种简单的电沉积方法,在不锈钢衬底上涂覆CoP电催化剂,作为酸性析氢反应(HER)的无pt替代催化剂电极。基于场发射扫描电镜(FE-SEM)分析,不同磷浓度对矿床形态特征有较大影响。在较低的磷浓度下,沉积物具有光滑的多孔结构。随着磷浓度的增加,表面结构逐渐变得粗糙,同时保持较高的孔隙度。利用x射线衍射(XRD)、能量色散x射线(EDX)和x射线光电子能谱(XPS)分析了催化剂的结构和组成特征。用电化学技术对所制备的催化剂的电催化活性进行了评价。提高的粗糙度有利于实现更大的电化学表面积,从而产生高的电催化活性。在10、50和100 mA cm−2电流密度下,优化后的包覆电极电催化活性最高,过电位值分别为128±1.2 mV、163±2.6 mV和181±4.3 mV。在三种不同的电流密度为10、50和100 mA cm - 2和100 mA cm - 2的情况下进行25小时的计时电位测量,CoP涂层电极表现出优异的稳定性。这些发现为研究使用非贵金属磷化物涂层不锈钢作为质子交换膜(PEM)水电解槽制氢的阴极电催化剂铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
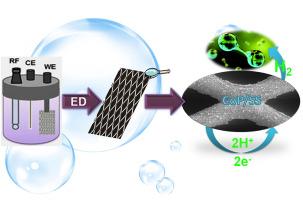
Porous CoP coating on stainless steel: Application as an electrocatalyst for acidic hydrogen evolution reaction
The development of new catalysts for the acidic hydrogen evolution reaction with both reasonable activity and stability is crucial to reducing the cost of PEM electrolyzers and promoting their widespread commercialization. In this study, we present a straightforward electrodeposition method for coating of CoP electrocatalyst on a stainless steel substrate as a Pt-free alternative catalyst electrode for the acidic hydrogen evolution reaction (HER). Based on field emission scanning electron microscopy (FE-SEM) analysis, varying the concentration of phosphorus has a substantial effect on the morphological features of the deposit. At lower phosphorus concentrations, the deposit has a smooth and porous structure. As the phosphorus concentration increases, the surface structure becomes gradually rough while maintaining a high level of porosity. The structural and compositional characteristics of the catalysts have been examined using X-ray diffraction (XRD), energy dispersive X-ray (EDX), and X-ray photoelectron spectroscopy (XPS) analyses. The electrocatalytic activity of the prepared catalysts was evaluated by electrochemical techniques. The elevated roughness was advantageous in achieving a greater electrochemical surface area resulting in high electrocatalytic activity. The optimized coated electrode exhibited the highest electrocatalytic activity, resulting in lower overpotential values of 128 ± 1.2 mV, 163 ± 2.6 mV, and 181 ± 4.3 mV at current densities of 10, 50, and 100 mA cm−2, respectively. The CoP coated electrode demonstrated exceptional stability based on the chronopotentiometric measurements conducted over a period of 25 h at three distinct current densities of 10, 50, and 100 mA cm−2 and 100 h at 100 mA cm−2. These findings pave the way to investigate the use of non-precious metallic phosphide coated stainless steel as cathode electrocatalysts for hydrogen production by proton exchange membrane (PEM) water electrolyzers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


