用于癌症放射治疗和免疫治疗的 PD-L1 siRNA 负载硼纳米粒子
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
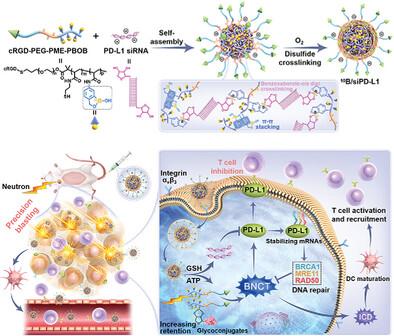
A PD-L1 siRNA-Loaded Boron Nanoparticle for Targeted Cancer Radiotherapy and Immunotherapy
Although the combination of radiotherapy and immunotherapy is regarded as a promising clinical treatment strategy, numerous clinical trials have failed to demonstrate synergistic effects. One of the key reasons is that conventional radiotherapies inevitably damage intratumoral effector immune cells. Boron Neutron Capture Therapy (BNCT) is a precise radiotherapy that selectively kills tumor cells while sparing adjacent normal cells, by utilizing 10B agents and neutron irradiation. Therefore, combinational BNCT-immunotherapy holds promise for achieving more effective synergistic effects. Here it develops a 10B-containing polymer that self-assembled with PD-L1 siRNA to form 10B/siPD-L1 nanoparticles for combinational BNCT-immunotherapy. Unlike antibodies, PD-L1 siRNA can inhibit intracellular PD-L1 upregulated by BNCT, activating T-cell immunity while also suppressing DNA repair. This can enhance BNCT-induced DNA damage, promoting immunogenic cell death (ICD) and further amplifying the antitumor immune effect. The results demonstrated that BNCT using 10B/siPD-L1 nanoparticles precisely killed tumor cells while sparing adjacent T cells and induced a potent antitumor immune response, inhibiting distal and metastatic tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


