钯催化的C-O键形成:芳基硼酸与o -亲电试剂的偶联
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于缺乏实际有用的o -亲电试剂,铃木型C-O耦合远不如公认的C-C耦合常见。在这项工作中,我们引入了环二酰基过氧化物作为与硼酸交叉偶联的有效的o亲电试剂,这一过程可能通过Pd(II)/Pd(IV)催化循环进行。该方法的成功可归因于环二酰基过氧化物具有较高的氧化电位,并且能够避免高价钯中间体中CO2的消除。此外,Pd(IV)与过氧化环二酰基配合物在醋酸盐和羧酸盐配体上保持基态单重态自旋,没有自由基特征,有利于金属转化步骤。该反应产生多种芳基酯,并附加羧基作为进一步功能化的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
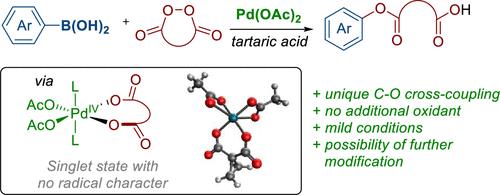
Pd-Catalyzed C–O Bond Formation: Coupling of Aryl Boronic Acids with O-Electrophiles
Due to the scarcity of practically useful O-electrophiles, Suzuki-type C–O coupling is far less common than the well-established C–C coupling. In this work, we introduce cyclic diacyl peroxides as effective O-electrophiles for cross-coupling with boronic acids, a process that likely proceeds via a Pd(II)/Pd(IV) catalytic cycle. The success of this method can be attributed to the higher oxidative potential of cyclic diacyl peroxides and the ability to avoid the elimination of CO2 in high-valent Pd intermediates. Additionally, the Pd(IV) complex with cyclic diacyl peroxide maintains a ground state singlet spin state with no radical character on the acetate and carboxylate ligands that facilitate the transmetalation step. The reaction produces a variety of aryl esters with an additional carboxylic acid group as a platform for further functionalization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


