草酸和硝酸在金红石型TiO2上共还原合成甘氨酸的研究
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
甘氨酸是日常生活中重要的氨基酸,但其传统合成往往涉及使用有毒化学品或催化剂。在温和的条件下,草酸和硝酸的电化学共还原提供了一种更环保的选择,但与单一催化剂相比具有挑战性。在此,我们在单一的低成本、无毒的金红石型TiO2/CNT (CNT =碳纳米管)上实现了甘氨酸的电合成。在合适的质子环境中,金红石型TiO2的独特性使甘氨酸的产率达到57%。草酸和硝酸在金红石型TiO2上被竞争性吸附,并在相同电位下选择性转化为乙醛酸和NH2OH。进一步在金红石型TiO2上进行固相C-N偶联和肟还原,高产出甘氨酸。密度泛函理论计算表明,在还原金红石型TiO2上适当的Ti-Ti距离有利于乙醛酸和NH2OH的解吸,以及硝酸盐还原初期含n物质的稳定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
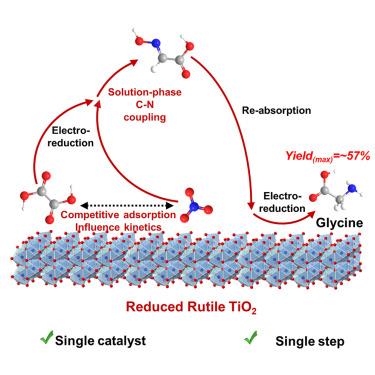
Glycine electrosynthesis by the co-reduction of oxalic and nitric acids on rutile TiO2
Glycine is an important amino acid for daily life, but its conventional synthesis often involves the use of toxic chemicals or catalysts. The electrochemical co-reduction of oxalic acid and nitric acid under mild conditions offers a greener alternative but is challenging over a single catalyst. Herein, we have realized this glycine electrosynthesis on a single low-cost and nontoxic rutile TiO2/CNT (CNT = carbon nanotube). The high glycine yield of 57% was made possible by the uniqueness of rutile TiO2 in the appropriate proton environment. Oxalic acid and nitric acid were competitively adsorbed on rutile TiO2 and selectively converted into glyoxylic acid and NH2OH at identical potentials. Further solution-phase C–N coupling and oxime reduction on rutile TiO2 produced glycine in high yields. Density functional theory calculations revealed that the appropriate Ti–Ti distance on the reduced rutile TiO2 fadvored the desorption of glyoxylic acid and NH2OH, as well as the stabilization of N-containing species at early-stage nitrate reduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


