双催化 C(sp2)-H 活化氮杂环,生成 C-N 异构体
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们描述了一种pdii催化的对映选择性C(杂芳基)-H活化方法,该方法由手性瞬态定向基团(cTDG)激活,以获得C - n反映体。含醛底物与手性氨基酸之间的可逆缩合有利于金属催化剂的配位和随后的atroopselective C-H活化。各种n -杂环,包括2-咪唑酮、吲哚、吡咯和2-吡啶酮,以及不同的烯烃偶联伙伴,以中等到良好的产率和对映选择性参与反应。这种方法的效用被证明了几个下游转化,迅速建立分子的复杂性。有机金属合成、H/D交换实验和密度泛函理论(DFT)计算揭示了cTDG在提高区域和atroo选择性方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
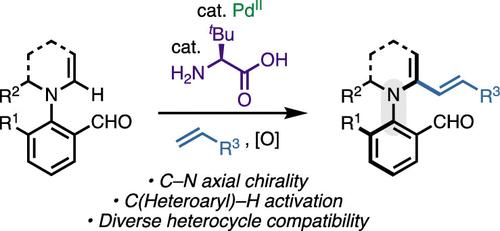
Dual Catalytic C(sp2)–H Activation of Azaheterocycles toward C–N Atropisomers
We describe a PdII-catalyzed enantioselective C(heteroaryl)–H activation method enabled by a chiral transient directing group (cTDG) to gain access to C–N atropisomers. Reversible condensation between the aldehyde-containing substrate and a chiral amino acid facilitates coordination of the metal catalyst and subsequent atroposelective C–H activation. Various N-heterocycles, including 2-imidazolone, indole, pyrrole, and 2-pyridone, and diverse alkene coupling partners participate in the reaction in moderate to good yields and enantioselectivity. The utility of this method is demonstrated by several downstream transformations that rapidly build up the molecular complexity. Organometallic synthesis, H/D exchange experiments, and density functional theory (DFT) calculations shed light on the critical role of the cTDG in enhancing the regio- and atroposelectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


