通过电化学原位红外光谱和理论模拟揭示金电极界面水的结构及其在酸性和碱性氢进化反应中的作用
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

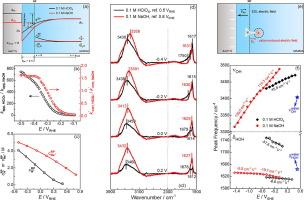
Unveiling the structure of interfacial water and its role in acidic and alkaline hydrogen evolution reaction at Au electrode by electrochemical in-situ infrared spectroscopy and theoretical simulation
Current for hydrogen evolution reaction (HER) in alkaline media has been generally found to be ca. one to two orders of magnitude smaller than that in acid. The origin is under hot debate. Different connectivity of the hydrogen bond networks in the electric double layer (EDL) has been recently proposed to play a significant role in the significant kinetic pH effect. To verify the generality of such an effect, the structure of Au/0.1 M HClO4 and Au/0.1 M NaOH interfaces and its correlation to HER kinetics have been investigated by cyclic voltammetry, electrochemical in-situ infrared spectroscopy and theoretical simulation. Our results reveal that, i) the current density and corresponding apparent rate constants for HER at Au/HClO4 are ca. 1 to 80 and 1 to 800 times higher than that at Au/NaOH interface at the same , respectively, while the intrinsic rate constants for acidic and alkaline HER estimated after properly considering the EDL effect are comparable; ii) at potentials negative of the potential of zero charge, there is an enrichment of H3O+ and Na+ near the surface, and the concentration of Na+ near Au surface is slightly higher than that of proton at the same due to more free excess charge at Au/NaOH interface. Partial desolvation of hydrated Na+ occurs to balance the excess free charge; iii) water structure at Au/NaOH interface is more heterogeneous than that at Au/HClO4 interface as evidenced by broader O–H stretching band with significant contribution at ca. 3590 cm−1; iv) the superposition of the Onsager field induced by the enriched cations with the field generated by applied electrode potential across the electric double layer leads to a significantly higher Stark tuning rate for the O–H stretching vibration in the alkaline HER range compared to that under acidic conditions; v) instead of the difference in the connectivity of hydrogen bond networks and the dynamics of water reorganization, smaller electrochemical driving force as a result of lower electric potential at the reaction plane is the origin for the smaller current as well as the apparent rate constant for HER at Au in alkaline media than that in acid under mild HER condition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


