IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
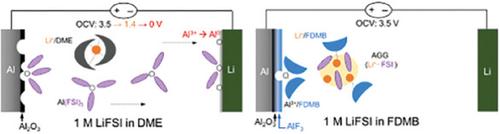
双(氟磺酰)亚胺锂(LiFSI)被广泛应用于锂金属电池,以形成稳定的氟化锂(LiF)基固体电解质相(SEI)。然而,氟化醚溶剂本身无法在铝(Al)集流体上形成保护性钝化层,从而导致 Al3⁺溶解和严重腐蚀。虽然氟化醚溶剂有望减轻铝腐蚀,但其机理仍不清楚。这里将介绍阳离子溶解和离子配对结构在缓解腐蚀中的作用。在 1 m LiFSI 中对 2,2,3,3-四氟-1,4-二甲氧基丁烷(FDMB)、1,1,2,2-四氟乙基-2,2,3,3-四氟丙基醚(TTE)/1,2-二甲氧基乙烷(DME)混合物和无氟醚进行了评估。在极端条件下(如 4.5 V vs Li/Li⁺,60 °C),FDMB 在防止腐蚀的同时促进了 AlF₃ 的形成。电化学和 DFT 分析表明,FDMB 与氧化铝表面产生的 Li⁺ 和 Al3⁺ 配合,发生了有利的脱氟反应。同时,Li+ 和 FSI- 之间形成的聚集离子对抑制了与 FSI- 配位的可溶性 Al3+ 物种的生成。用烷基链修饰 FDMB 可降低 Al3+ 物种的溶解度,从而进一步增强抗腐蚀效果。相比之下,DME/TTE 与四甘醇二甲醚 (TEGDME) 类似,表现出更多的铝腐蚀,这是因为 Li+ 和 Al3+ 在 TTE 上的溶解有限,不利于脱氟。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effects of Li+ Solvation Structures on Aluminum Corrosion in Ether-Based Electrolyte Solutions with Lithium Bis(Fluorosulfonyl)imide (LiFSI)
Lithium bis(fluorosulfonyl)imide (LiFSI) is widely used in lithium-metal batteries to form a stable lithium fluoride (LiF)-based solid electrolyte interphase (SEI). However, the FSI⁻ itself fails to create a protective passivation layer on aluminum (Al) current collectors, leading to Al3⁺ dissolution and severe corrosion. While fluorinated ether solvents have shown promise in mitigating Al corrosion, the mechanisms remain unclear. Here, the role of cation solvations and ion pairing structures is shown in corrosion mitigation. 2,2,3,3-tetrafluoro-1,4-dimethoxybutane (FDMB), a 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE)/1,2-dimethoxyethane (DME) mixture, and non-fluorinated ethers are evaluated in 1 m LiFSI. FDMB promoted the formation of AlF₃ while preventing corrosion under extreme conditions (e.g., 4.5 V vs Li/Li⁺, 60 °C). Electrochemical and DFT analyses showed that FDMB underwent favorable defluorination in coordination with both Li⁺ and Al3⁺ that arose from the oxidizing Al surface. Meanwhile, the formation of aggregated ion pairs between Li+ and FSI⁻ inhibited the generation of soluble Al3+ species coordinated with FSI−. Modifying FDMB with alkyl chains further enhanced the anti-corrosive effects by reducing the solubility of Al3+ species. In contrast, DME/TTE exhibited more Al corrosion, similar to tetraethylene glycol dimethyl ether (TEGDME), due to less favorable defluorination by the limited solvation of Li+ and Al3+ on TTE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


