高倍率锂金属电池用不对称醚溶剂
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
最近,基于削弱锂离子溶解的电解质溶剂设计在提高锂金属电池的循环性能方面大有可为。然而,它们往往面临着氧化还原动力学缓慢和高倍率循环可逆性差的问题。在此,我们报告了使用不对称溶剂分子可大大加快锂离子氧化还原动力学。与对称醚相比,不对称醚(1-乙氧基-2-甲氧基乙烷、1-甲氧基-2-丙氧基乙烷)显示出更高的交换电流密度,并增强了高速锂0电镀/剥离的可逆性。调整氟化程度可进一步提高氧化稳定性和锂0的可逆性。含有 2 M 双(氟磺酰)亚胺锂的不对称 1-(2,2,2-三氟)-乙氧基-2-甲氧基乙烷表现出高交换电流密度、氧化稳定性和紧凑的固体-电解质相(约 10 nm)。这种电解质在最先进的电解质中表现出卓越的性能,在高倍率锂(50 μm)||LiNi0.8Mn0.1Co0.1O2(NMC811,4.9 mAh cm-2)电池中实现了超过 220 次循环,并首次在无阳极铜||Ni95 袋式电池(200 mAh)中,在电动垂直起落循环协议下实现了超过 600 次循环。我们在不对称分子设计策略方面的发现为实现高功率锂金属电池的快速氧化还原动力学指出了一条新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。

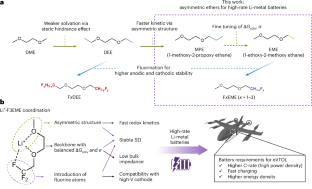
Asymmetric ether solvents for high-rate lithium metal batteries
Recent electrolyte solvent design based on weakening lithium-ion solvation have shown promise in enhancing cycling performance of Li-metal batteries. However, they often face slow redox kinetics and poor cycling reversibility at high rate. Here we report using asymmetric solvent molecules substantially accelerates Li redox kinetics. Asymmetric ethers (1-ethoxy-2-methoxyethane, 1-methoxy-2-propoxyethane) showed higher exchange current densities and enhanced high-rate Li0 plating/stripping reversibility compared to symmetric ethers. Adjusting fluorination levels further improved oxidative stability and Li0 reversibility. The asymmetric 1-(2,2,2-trifluoro)-ethoxy-2-methoxyethane, with 2 M lithium bis(fluorosulfonyl)imide, exhibited high exchange current density, oxidative stability, compact solid–electrolyte interphase (~10 nm). This electrolyte exhibited superior performance among state-of-the-art electrolytes, enabling over 220 cycles in high-rate Li (50 μm)||LiNi0.8Mn0.1Co0.1O2 (NMC811, 4.9 mAh cm−2) cells and for the first time over 600 cycles in anode-free Cu | |Ni95 pouch cells (200 mAh) under electric vertical take-off and landing cycling protocols. Our findings on asymmetric molecular design strategy points to a new pathway towards achieving fast redox kinetics for high-power Li-metal batteries. There is growing interest in designing electrolytes to enable Li-metal batteries. Here the authors show that asymmetric solvents improve lithium redox kinetics and achieve long cycle life in anode-free cells under electric vertical take-off and landing conditions, demonstrating potential for future high-power applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


