铜(I)催化炔基糖苷的偶联:丁烷-1,3-二炔连接双糖和二核苷的合成。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了铜催化炔苷偶联促进丁-1,3-二炔连接二糖合成的情况。该反应的特点是采用环保试剂,条件温和,因此所需产物的收率很高。这种方法对官能团的耐受性强,底物范围广。此外,我们还成功地将这种方法扩展到丁-1,3-二炔连接二核苷的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
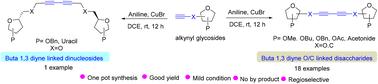
Copper(i)-catalyzed coupling of alkynyl glycosides: synthesis of buta-1,3-diyne-linked disaccharides and dinucleosides†
Herein we report copper-catalyzed coupling of alkynyl glycosides facilitating the synthesis of buta-1,3-diyne-linked disaccharides. The reaction is characterized by mild conditions employing eco-friendly reagents, resulting in good yields of the desired products. This methodology exhibits significant functional group tolerance with broad substrate scope. Furthermore, we have successfully extended this approach to the synthesis of buta-1,3-diyne-linked dinucleosides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


