吡啶-硼基自由基使对喹啉酯芳构化的自由基前体。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-28
Epub Date: 2025-02-13
DOI:10.1021/acs.joc.4c02831
引用次数: 0
摘要
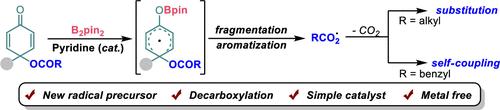
成功开发了一类前芳香族羧酸对醌酯自由基前体,在吡啶和二硼试剂存在下,通过吡啶-硼自由基诱导的芳香化作用,对醌酯的对位 C-O 键可发生均解裂解,并通过羧基原位脱羧得到相应的烷基自由基。此外,芳香族自由基前体还可进一步用于苯磺酰基化合物和自由基自偶联的自由基取代。这种方法不仅提供了一种生成自由基中间体的新方法,而且还扩大了硼自由基的应用范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Radical Precursor Based on the Aromatization of p-Quinol Esters Enabled by Pyridine-Boryl Radical.
A class of prearomatic carboxylic acid p-quinol ester radical precursors has been developed successfully, which could undergo homolytic cleavage of the para C-O bond of p-quinol esters via pyridine-boryl radical-induced aromatization in the presence of pyridines and diboron reagents, affording the corresponding alkyl radical via decarboxylation from the carboxyl radical in situ. In addition, the prearomatic radical precursors were further applied in radical substitution with phenylsulfonyl compounds and radical self-coulpings. This method not only provides a new approach to the generation of a radical intermediate but also expands the application of boron radicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


