针刺素A、B、D、E、F的集体全合成
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-05
DOI:10.1021/acs.joc.4c0302410.1021/acs.joc.4c03024
引用次数: 0
摘要
提出了一种集体收敛方法,用于对旋选择性全合成aculeatins A, B, D, E和F,具有[3 + 2]-环加成,铁介导的还原性N-O键裂解和级联螺旋环化。此外,这一简短的六步策略在合成非天然类似物中得到了补充,如6-外稃D、6-外稃E和6-外稃F。本文章由计算机程序翻译,如有差异,请以英文原文为准。
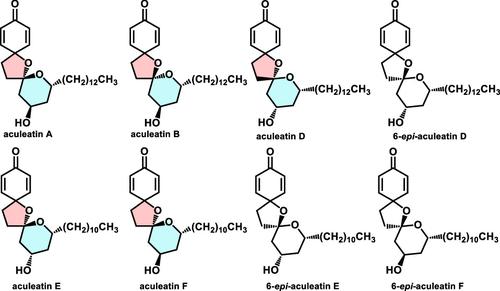
Collective Total Synthesis of Aculeatin A, B, D, E, and F
A collective convergent approach for the enantioselective total synthesis of aculeatins A, B, D, E, and F is presented, featuring [3 + 2]-cycloaddition, iron-mediated reductive N–O bond cleavage, and cascade spirocyclization. Moreover, this short six-step strategy is supplemented in synthesizing unnatural analogs of aculeatins such as 6-epi-aculeatin D, 6-epi-aculeatin E, and 6-epi-aculeatin F.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


