工程纳米囊泡用于MRSA感染引起的深度骨髓炎的精确和无创治疗,增强免疫反应
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
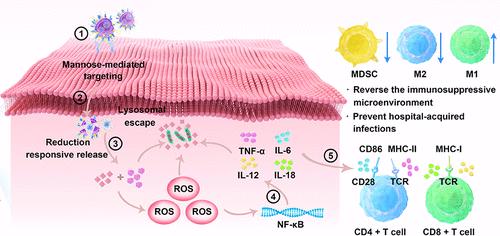
耐甲氧西林金黄色葡萄球菌(MRSA)引起的医院获得性持续性骨髓炎的临床治疗面临两个主要挑战:深层组织药物递送无效和对抗免疫抑制微环境的快速建立。事实上,MRSA可以逃避免疫监视,破坏先天和适应性免疫反应。在本研究中,通过结合声动力疗法(SDT)和免疫调节,构建了工程纳米囊泡,用于精确和无创地去除深层组织中的MRSA,并使用新工程纳米囊泡激活抗菌免疫反应。巨噬细胞来源的M1表型微泡(M1- mw)内化万古霉素交联胶束与声敏剂吲哚青绿(ICG) (VCG胶束)。将M1-MW的囊泡与聚乙二醇化的甘露糖嫁接,以便在感染部位靶向积累。超声暴露后,VCG胶束对高还原环境有反应,释放ICG产生ROS。这种效果与万古霉素的存在相结合,杀死了MRSA。在骨髓炎感染模型中,我们观察到巨噬细胞的存活率提高,巨噬细胞重编程为促炎M1表型。后者促进t细胞活化和免疫防御mrsa伪装的同源细胞转移感染。因此,我们的研究提出了一种非侵入性和有效的治疗深部骨髓炎的方法(VCG@MMW),可以提高细菌清除率,降低复发风险,增强免疫反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineered Nanovesicles for the Precise and Noninvasive Treatment of Deep Osteomyelitis Caused by MRSA Infection with Enhanced Immune Response
The clinical treatment of hospital-acquired persistent osteomyelitis caused by methicillin-resistant Staphylococcus aureus (MRSA) presents two major challenges: ineffective drug delivery into deep tissues and counteracting the rapid establishment of an immunosuppressive microenvironment. Indeed, MRSA can evade immunosurveillance and undermine both innate and adaptive immune responses. Herein, the engineered nanovesicles, functioning by combining sonodynamic therapy (SDT) with immune modulation, were constructed for the precise and noninvasive removal of MRSA in deep tissue and activation of the antimicrobial immune response using a newly engineered nanovesicle. Macrophage-derived M1 phenotypic microvesicles (M1-MW) internalized vancomycin-cross-linked micelles with the acoustic sensitizer indocyanine green (ICG) (VCG micelles). The vesicles of M1-MW were grafted with PEGylated mannose, allowing for targeted accumulation at the infection site. The VCG micelles were responsive to the highly reducing environment and released ICG to generate ROS after exposure to ultrasounds. This effect was combined with the presence of vancomycin to kill MRSA. In an osteomyelitis infection model, we observed an improved survival rate and reprogramming of macrophages to a pro-inflammatory M1 phenotype. The latter promoted T-cell activation and immune defense against MRSA-camouflaged homologous cell-transferred infections. Thus, our study presents a noninvasive and efficient treatment (VCG@MMW) for deep osteomyelitis with improved bacterial clearance and reduced risk of recurrence with enhanced immune response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


