冷暴露疗法增强单原子纳米酶介导的癌症疫苗治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
单原子纳米酶由于其优异的过氧化物酶活性和良好的生物相容性,在肿瘤疫苗(TV)的制备中非常有效。然而,肿瘤内的免疫抑制环境会降低这些疫苗的效力。冷暴露(CE)治疗是一种无创、直接的抗肿瘤方法,不仅可以抑制肿瘤代谢,还可以改善免疫抑制的肿瘤环境。在本研究中,我们利用铜单原子纳米酶(Cu SAZ)开发了个性化的TV,并通过引入CE来增强其长期抗肿瘤效果。我们通过高温碳化初步合成了Cu SAZ,该材料具有良好的POD活性和光热特性。在808 nm激光照射下,纳米酶产生活性氧(ROS)和热量,诱导4T1乳腺癌细胞或CT26结肠癌细胞的免疫原性细胞死亡,促进TV的产生。在我们的体内肿瘤防治模型中,我们注意到CE显著提高了TV的疗效。其主要机制涉及CE能够降低髓源性抑制细胞(MDSCs)的比例,降低肿瘤细胞中的葡萄糖代谢,增加CD8+ T细胞和记忆T细胞的比例。总的来说,我们的发现为设计创新的电视系统提供了有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
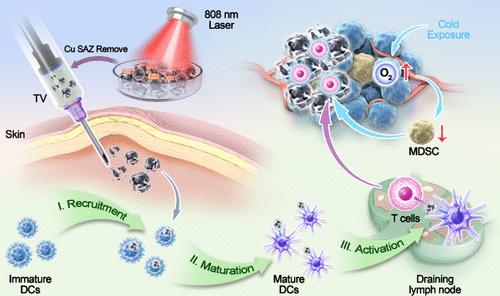
Cold Exposure Therapy Enhances Single-Atom Nanozyme-Mediated Cancer Vaccine Therapy
Single-atom nanozymes are highly effective in the preparation of tumor vaccines (TV) due to their superior peroxidase (POD) activity and excellent biocompatibility. However, the immunosuppressive environment within tumors can diminish the efficacy of these vaccines. Cold exposure (CE) therapy, a noninvasive and straightforward antitumor method, not only suppresses tumor metabolism but also ameliorates the immunosuppressive tumor milieu. In this study, we developed personalized TV using copper single-atom nanozyme (Cu SAZ) and enhanced their long-term antitumor efficacy by introducing CE. We initially synthesized the Cu SAZ via high-temperature carbonization, which demonstrated robust POD activity and photothermal characteristics. Upon exposure to 808 nm laser irradiation, the nanozyme generated reactive oxygen species (ROS) and heat, inducing immunogenic cell death in 4T1 breast cancer cells or CT26 colon cancer cells and facilitating TV production. In our in vivo tumor prevention and treatment model, we noted that CE significantly boosted the efficacy of the TV. The primary mechanism involves CE’s ability to lower the ratio of myeloid-derived suppressor cells (MDSCs), decrease glucose metabolism in tumor cells, and increase the proportions of CD8+ T cells and memory T cells. Collectively, our findings offer promising avenues for designing innovative TV systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


