人类诱导多能干细胞衍生产品用于帕金森病自体细胞治疗的临床前安全性和有效性
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
人类诱导多能干细胞(hiPSC)衍生的中脑多巴胺能细胞(mDACs)是帕金森病(PD)自体细胞治疗的一个有希望的来源,但标准化的监管标准对临床转化至关重要。在这项临床前研究中,我们使用外源性重编程技术,从4名散发性PD患者新鲜活检的成纤维细胞中产生了多个临床级的hiPSC细胞系,并使用精炼的21天方案将其分化为mdac。严格的评估包括全基因组/外显子组测序、RNA测序和体内研究,包括一项为期39周的符合良好实验室规范的小鼠安全性研究。虽然来自所有品系的mDACs均符合安全标准,但来自一名患者的mDACs未能改善啮齿动物的行为结果,这强调了个体间的可变性。重要的是,体外评估不能可靠地预测体内疗效,将多巴胺能纤维密度确定为关键疗效标准。这些发现支持了自体细胞治疗的全面质量控制指南,并为8例散发性PD患者的临床试验铺平了道路,该试验计划于2025年开始。本文章由计算机程序翻译,如有差异,请以英文原文为准。
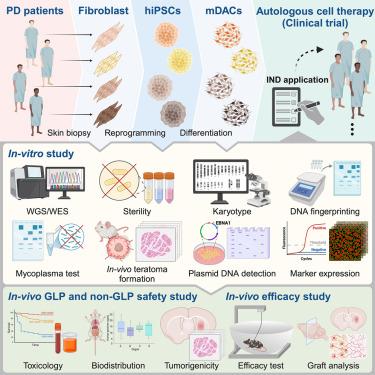
Pre-clinical safety and efficacy of human induced pluripotent stem cell-derived products for autologous cell therapy in Parkinson’s disease
Human induced pluripotent stem cell (hiPSC)-derived midbrain dopaminergic cells (mDACs) represent a promising source for autologous cell therapy in Parkinson’s disease (PD), but standardized regulatory criteria are essential for clinical translation. In this pre-clinical study, we generated multiple clinical-grade hiPSC lines from freshly biopsied fibroblasts of four sporadic PD patients using episomal reprogramming and differentiated them into mDACs using a refined 21-day protocol. Rigorous evaluations included whole-genome/exome sequencing, RNA sequencing, and in vivo studies, including a 39-week Good Laboratory Practice-compliant mouse safety study. While mDACs from all lines met safety criteria, mDACs from one patient failed to improve rodent behavioral outcomes, underscoring inter-individual variability. Importantly, in vitro assessments did not reliably predict in vivo efficacy, identifying dopaminergic fiber density as a key efficacy criterion. These findings support comprehensive quality control guidelines for autologous cell therapy and pave the way for a clinical trial with eight sporadic PD patients, scheduled to commence in 2025.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


