通过模拟异种移植排斥反应中抗原的处理和呈递,将疫苗靶向树突状细胞
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
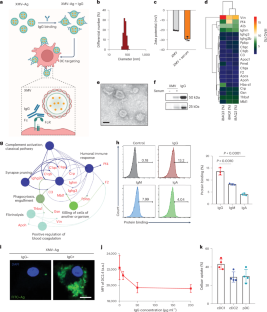
将疫苗靶向递送到树突状细胞(dc)是一项挑战。本研究表明,通过模拟异种移植组织排斥反应期间发生的快速和强烈的抗原加工和呈递,可以利用暴露组织特异性抗体的异种细胞膜源性囊泡将肽抗原和mrna编码抗原递送到dc。在患有小鼠黑色素瘤和胸腺瘤的小鼠中,包被肿瘤来源的抗原肽或包被包被肿瘤抗原mRNA编码的脂质纳米颗粒的异种囊泡可引发有效的肿瘤特异性t细胞反应,从而抑制肿瘤生长。用包裹编码严重急性呼吸综合征冠状病毒2刺突蛋白的mRNA的异种囊泡包被的脂质纳米颗粒免疫小鼠,可诱导抗刺突受体结合域免疫球蛋白G和中和抗体的滴度,分别约为商品化mRNA -脂质纳米颗粒疫苗诱导的滴度的32倍和6倍。模拟异种囊泡上的免疫球蛋白G和树突状细胞上的片段结晶性受体之间的生物识别的优势,可能证明在人类中使用动物源性生物制品的安全风险评估是合理的。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Targeting vaccines to dendritic cells by mimicking the processing and presentation of antigens in xenotransplant rejection
Targeting the delivery of vaccines to dendritic cells (DCs) is challenging. Here we show that, by mimicking the fast and strong antigen processing and presentation that occurs during the rejection of xenotransplanted tissue, xenogeneic cell membrane-derived vesicles exposing tissue-specific antibodies can be leveraged to deliver peptide antigens and mRNA-encoded antigens to DCs. In mice with murine melanoma and murine thymoma, xenogeneic vesicles encapsulating a tumour-derived antigenic peptide or coated on lipid nanoparticles encapsulating an mRNA coding for a tumour antigen elicited potent tumour-specific T-cell responses that inhibited tumour growth. Mice immunized with xenogeneic vesicle-coated lipid nanoparticles encapsulating an mRNA encoding for the spike protein of severe acute respiratory syndrome coronavirus 2 elicited titres of anti-spike receptor-binding domain immunoglobulin G and of neutralizing antibodies that were approximately 32-fold and 6-fold, respectively, those elicited by a commercialized mRNA–lipid nanoparticle vaccine. The advantages of mimicking the biological recognition between immunoglobulin G on xenogeneic vesicles and fragment crystallizable receptors on DCs may justify the assessment of the safety risks of using animal-derived biological products in humans. By mimicking antigen processing and presentation during the rejection of xenotransplanted tissue, xenogeneic cell membrane-derived vesicles encapsulating peptide antigens or mRNA-encoded antigens function as vaccines targeted to dendritic cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


