界面柔韧性控制着超分子网络的成核和生长
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
超分子网络在自然界中大量存在,并且像晶体材料一样,通常从最初的成核位置发展,然后基于组分之间的定向相互作用而生长。传统上,结合强度和相互作用的方向性被认为决定了成核和晶体生长,而结构柔韧性有利于缺陷。通常,高分子单体存在多个具有相对分子内灵活性的结合位点,但这种灵活性对调节网络形成的影响很少受到关注。在这里,我们引入了“界面柔性”的概念,并证明了它在超分子网络的成核和生长中的关键重要性。作为一个模型系统,我们使用了三对称的基于dna的大单体,它们通过其尖端的弱π -π相互作用组织成六边形网络。π -π相互作用的方向性和低空间容差意味着取向的微小变化对有效价的影响很大。我们表明,无论亲和度如何,过多的界面灵活性都会破坏网络的形成。调整界面灵活性大大扩展了合成超分子材料的可用设计空间。本文章由计算机程序翻译,如有差异,请以英文原文为准。

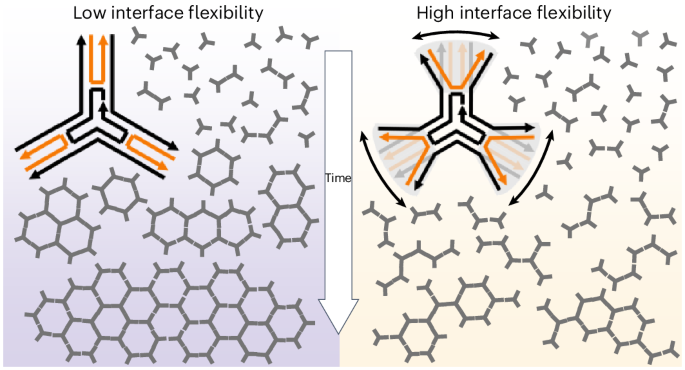
Interface flexibility controls the nucleation and growth of supramolecular networks
Supramolecular networks are abundantly present in nature and, like crystalline materials, often develop from an initial nucleation site, followed by growth based on directional interactions between components. Traditionally, the binding strength and directionality of interactions is thought to dictate nucleation and crystal growth, whereas structural flexibility favours defects. Usually, macromonomers present multiple binding sites with relative intramolecular flexibility, but the effects of such flexibility on regulating network formation have been given little attention. Here we introduce the concept of ‘interface flexibility’ and demonstrate its critical importance in the nucleation and growth of supramolecular networks. As a model system, we use trisymmetric DNA-based macromonomers, which organize into hexagonal networks through weak π–π interactions at their tips. The directional nature and low spatial tolerance of π–π interactions mean that small shifts in orientation have a large effect on effective valency. We show that too much interface flexibility disrupts network formation, regardless of affinity. Tuning the interface flexibility greatly expands the available design space for synthetic supramolecular materials. Supramolecular ordered networks are formed through directional interactions of uniform macromonomer building blocks. Now it has been shown that, rather than intermolecular affinity, the flexibility of the binding interface (‘interface flexibility’) dominates the mechanism of self-assembly. This study provides an intuitive understanding of the role of interface flexibility in supramolecular self-assembly.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


