植物辅助NLR的激活和抑制机制
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
植物核苷酸结合的富亮氨酸重复序列(NLR)受体感知病原体效应物并形成抵抗体以赋予免疫。一些传感器NLR抵抗体产生小分子,诱导与两种脂酶样蛋白EDS1和SAG101以及称为NRG1的辅助NLR形成异源三聚体复合物(参考文献)。2、3)。传感器NLR抗性小体的激活也会触发NRG1寡聚化和质膜上抗性小体的形成4,5。我们证明拟南芥AtEDS1-AtSAG101-AtNRG1A异源三聚体的形成被AtNRG1A寡聚缺失突变体l134e5,6所稳定。我们报道了具有相似组装机制的AtEDS1-AtSAG101-AtNRG1A L134E和AtEDS1-AtSAG101-AtNRG1C异源三聚体的结构。AtNRG1A信号通过与AtEDS1-AtSAG101异源二聚体及其小分子配体的相互作用而激活。截断的AtNRG1C维持了AtNRG1A的核心相互作用域,但与AtEDS1-AtSAG101进一步相互作用,以胜过AtNRG1A。此外,AtNRG1C缺乏一个n端信号域,并显示核质定位,这有利于其对同样是核质的AtEDS1-AtSAG101的隔离。我们的研究揭示了植物辅助植物NLR的激活和抑制机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
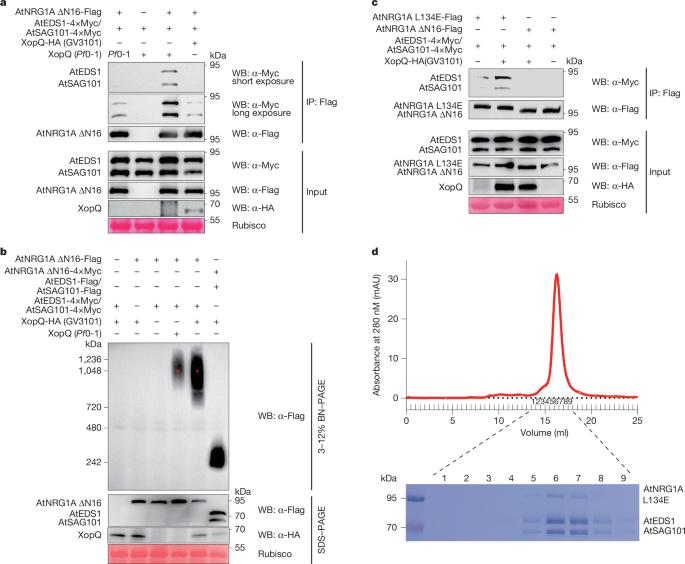
Activation and inhibition mechanisms of a plant helper NLR
Plant nucleotide-binding leucine-rich repeat (NLR) receptors sense pathogen effectors and form resistosomes to confer immunity1. Some sensor NLR resistosomes produce small molecules to induce formation of a heterotrimer complex with two lipase-like proteins, EDS1 and SAG101, and a helper NLR called NRG1 (refs. 2,3). Activation of sensor NLR resistosomes also triggers NRG1 oligomerization and resistosome formation at the plasma membrane4,5. We demonstrate that the Arabidopsis AtEDS1–AtSAG101–AtNRG1A heterotrimer formation is stabilized by the AtNRG1A loss-of-oligomerization mutant L134E5,6. We report structures of AtEDS1–AtSAG101–AtNRG1A L134E and AtEDS1–AtSAG101–AtNRG1C heterotrimers with similar assembly mechanisms. AtNRG1A signalling is activated by the interaction with the AtEDS1–AtSAG101 heterodimer in complex with their small-molecule ligand. The truncated AtNRG1C maintains core interacting domains of AtNRG1A but develops further interactions with AtEDS1–AtSAG101 to outcompete AtNRG1A. Moreover, AtNRG1C lacks an N-terminal signalling domain and shows nucleocytoplasmic localization, facilitating its sequestration of AtEDS1–AtSAG101, which is also nucleocytoplasmic. Our study shows the activation and inhibition mechanisms of a plant helper NLR. In plant immunity, sensor NLR resistosomes can generate heterotrimer complexes comprising the helper NLR NRG1, and investigation of cryo-EM structures shows the mechanisms of these heterotrimers in immune activation and suppression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


