皮质杏仁核在形成社会相遇中的关键作用
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
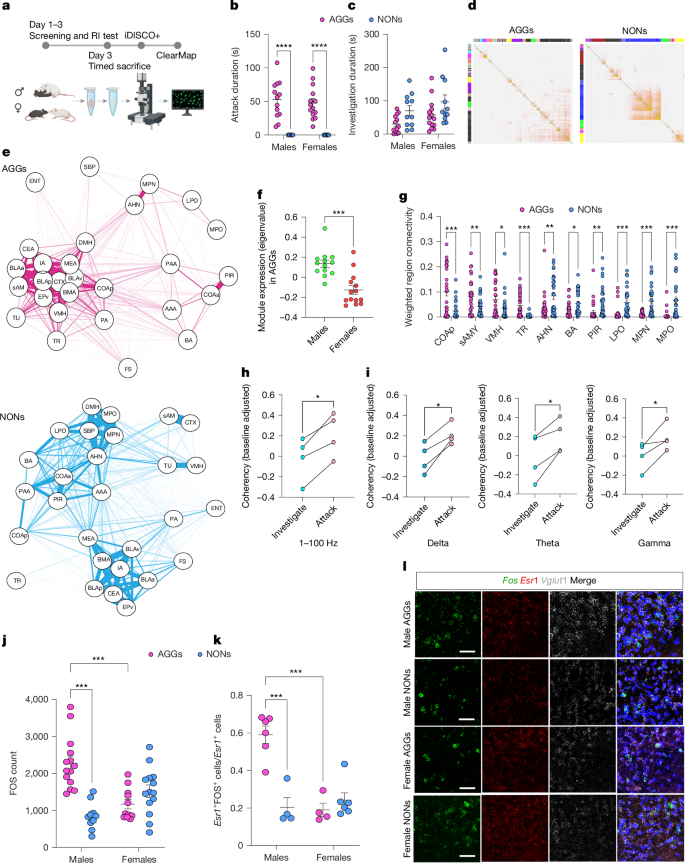
侵略是一种进化上保守的行为,它控制着社会等级,保护着宝贵的资源。在老鼠身上,攻击性行为可以分为食欲阶段,包括接近和调查,以及完善阶段,包括咬、踢和摔跤。在这里,通过对小鼠全脑FOS表达进行无监督加权相关网络分析,我们确定了一组大脑区域,包括下丘脑和杏仁核亚区以及嗅觉皮质区域,这些区域在雄性攻击者中高度共激活,而在雌性攻击者中则没有。后外侧皮质杏仁核(COApl)——一个扩展的嗅觉结构——被发现是一个中枢区域,基于与集群中其他区域相关的数量和强度。我们的数据还表明,仅在雄性小鼠中,COApl (COAplEsr1)中表达雌激素受体1 (Esr1)的细胞在攻击行为和攻击前的调查期间表现出增加的活性。COAplEsr1细胞在雄性攻击者体内的化学遗传或光遗传抑制可减少攻击并增加亲社会调查,而不影响社会奖励和强化行为。我们进一步表明,COAplEsr1投射到下丘脑腹内侧和中央杏仁核是这些行为所必需的。总的来说,这些数据表明,在具有攻击性的雄性中,coapplesr1细胞对社会刺激有特异性反应,从而增强了它们的显著性并促进了攻击行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。

A crucial role for the cortical amygdala in shaping social encounters
Aggression is an evolutionarily conserved behaviour that controls social hierarchies and protects valuable resources. In mice, aggressive behaviour can be broken down into an appetitive phase, which involves approach and investigation, and a consummatory phase, which involves biting, kicking and wrestling1. Here, by performing an unsupervised weighted correlation network analysis on whole-brain FOS expression in mice, we identify a cluster of brain regions, including hypothalamic and amygdalar subregions and olfactory cortical regions, that are highly co-activated in male but not in female aggressors. The posterolateral cortical amygdala (COApl)—an extended olfactory structure—was found to be a hub region, on the basis of the number and strength of correlations with other regions in the cluster. Our data also show that oestrogen receptor 1 (Esr1)-expressing cells in the COApl (COAplEsr1) exhibit increased activity during attack behaviour and during bouts of investigation that precede an attack, in male mice only. Chemogenetic or optogenetic inhibition of COAplEsr1 cells in male aggressors reduces aggression and increases pro-social investigation without affecting social reward and reinforcement behaviour. We further show that COAplEsr1 projections to the ventromedial hypothalamus and central amygdala are necessary for these behaviours. Collectively, these data suggest that, in aggressive males, COAplEsr1 cells respond specifically to social stimuli, thereby enhancing their salience and promoting attack behaviour. The posterolateral cortical amygdala and other connected brain regions have a key role in mediating the transition from investigative to aggressive behaviour in male mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


