甲苯达唑通过靶向TUBA1A诱导ZBP-1介导的急性髓系白血病细胞PANoptosis,发挥抗白血病作用
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
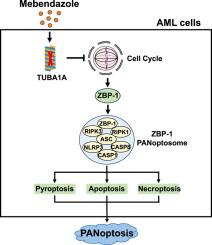
尽管近年来AML治疗取得了显著进展,但相当一部分患者仍然难治性或复发风险高,疗效有限。因此,开发治疗急性髓性白血病的新药迫在眉睫。方法利用小分子药物文库筛选引起AML细胞炎性死亡的药物。采用细胞活力、细胞形态学分析、western blotting、RNA-seq等方法,探讨咪唑(MBD)诱导AML细胞死亡的途径。利用细胞周期分析、蛋白表达谱分析、分子对接、western blotting和慢病毒过表达等方法分析AML细胞MBD靶蛋白。利用AML细胞系和患者源性原代AML细胞构建的肿瘤异种移植模型,评估MBD在体内的抗AML活性。结果在本研究中,我们已经确定了咪苯达唑(MBD),一种以其低毒和低成本而闻名的传统驱虫药,作为一种有效的体外抗aml作用的药物。此外,我们在异种移植小鼠模型中观察到其对AML细胞系和原代AML细胞的侵袭有抑制作用,同时注意到其在正常小鼠体内的毒副作用可以忽略不计。机制上,MBD通过抑制微管蛋白α1A (TUBA1A)抑制G2/M期细胞周期,促进ZBP-1介导的AML细胞PANoptosis。我们的研究结果证实MBD在临床前模型中具有抗aml活性。结论MBD具有显著的临床转化潜力,为AML患者提供了新的潜在药物。此外,TUBA1A可作为TUBA1A异常表达肿瘤的潜在新治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mebendazole induces ZBP-1 mediated PANoptosis of acute myeloid leukemia cells by targeting TUBA1A and exerts antileukemia effect
Background
Despite notable advancements in AML therapy in recent years, a substantial proportion of patients remain refractory or at high risk of recurrence with limited efficacy. Therefore, it’s urgent to develop novel drugs for treating AML.Methods
The small molecule drug library was utilized to screen for drugs that elicit the inflammatory death of AML cells. Cell viability, cell morphological analysis, western blotting, and RNA-seq were used to determine the pathway of Mebendazole (MBD)-induced AML cell death. Cell cycle analysis, protein expression profiling, molecular docking, western blotting and lentivirus overexpression were used to analyze the target protein of MBD in AML cells. The anti-AML activity of MBD in vivo was evaluated using tumor xenograft models constructed by AML cell lines and patient-derived primary AML cells.Results
In this study, we have identified Mebendazole (MBD), a conventional anthelmintic drug known for its low toxicity and cost, as a potent agent that exerts significant anti-AML effects in vitro. Furthermore, we have observed its inhibitory effects on the invasion of AML cell lines and primary AML cells in xenograft mouse models, while noting its negligible toxic side effects in normal mice in vivo. Mechanically, MBD inhibits the cell cycle in G2/M phase by inhibiting tubulin α1A (TUBA1A) and promotes ZBP-1 mediated PANoptosis in AML cells. Our results confirm that MBD exerts anti-AML activity in preclinical models.Conclusion
These results highlight the remarkable clinical translational potential of MBD, providing new potential medicine for AML patients. In addition, TUBA1A can be used potential novel therapeutic target in tumors with abnormal TUBA1A expression.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


