listin促进α-突触核蛋白降解,通过ESCRT途径缓解帕金森病
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-12
引用次数: 0
摘要
帕金森病(PD)是一种神经退行性疾病,其特征是大脑黑质区域多巴胺能神经元内异常α-突触核蛋白(α-syn)的进行性积累。尽管α-syn的过度积累是PD发病的关键,但其清除的机制尚不明确。在这项研究中,我们发现运输所需的内体分选复合物(ESCRT)系统在捕获和促进泛素化α-syn的降解中起着至关重要的作用。发现E3泛素连接酶Listerin可促进k27连接的α-syn多泛素化,将其引导至核内体进行随后的降解。我们发现,在PD小鼠模型中,Listerin基因的缺失加剧了神经退行性进展,而Listerin的过表达有效地减轻了PD小鼠的疾病进展。因此,我们的研究揭示了α-syn降解的机制,并确定了Listerin是治疗PD的有希望的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
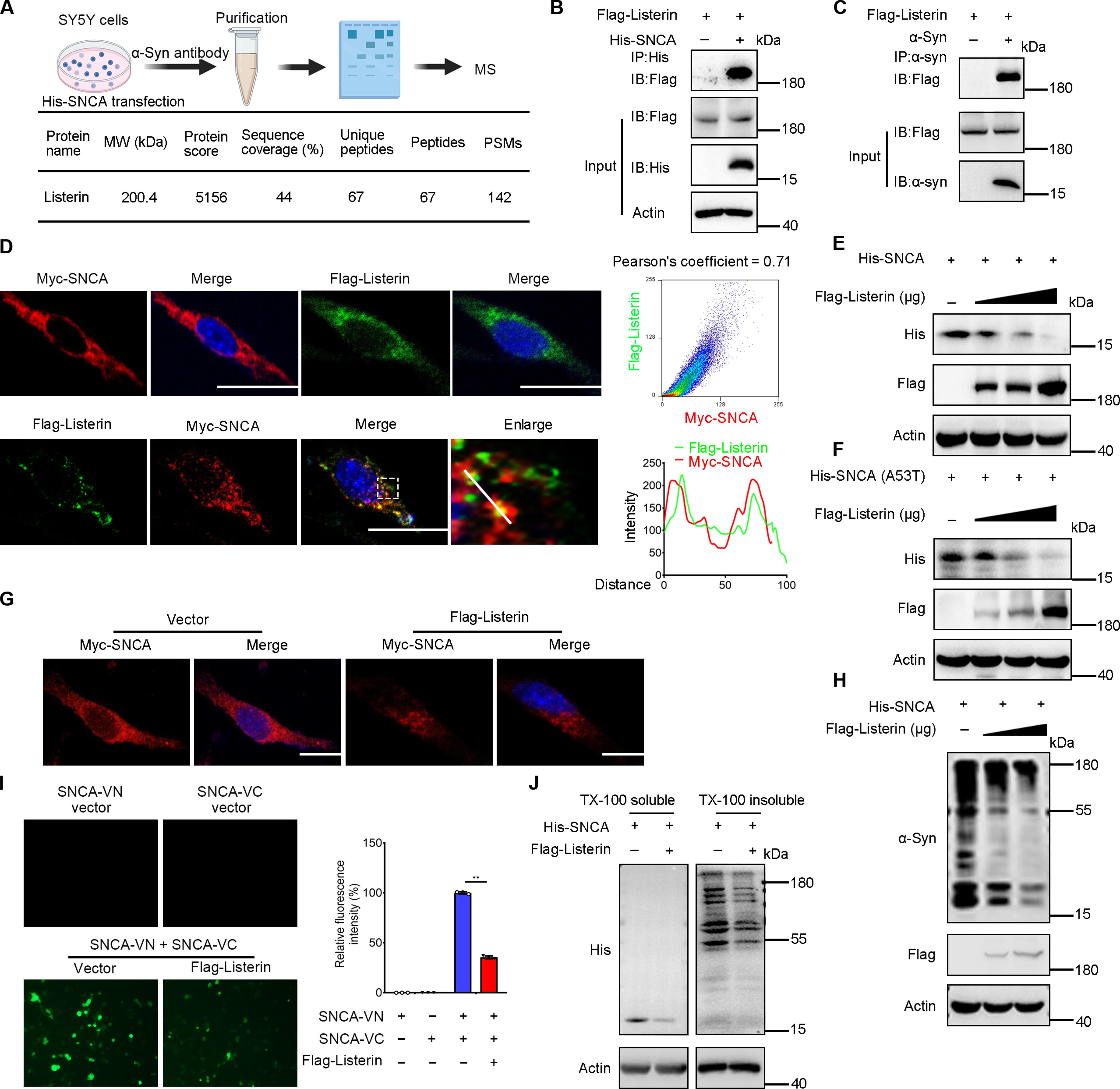
Listerin promotes α-synuclein degradation to alleviate Parkinson’s disease through the ESCRT pathway
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the progressive accumulation of abnormal α-synuclein (α-syn) within dopaminergic neurons in the substantia nigra region of the brain. Despite excessive accumulation of α-syn being key to the pathogenesis of PD, the mechanisms governing its clearance remain elusive. In this study, we found that the endosomal sorting complex required for transport (ESCRT) system plays a crucial role in capturing and facilitating the degradation of ubiquitinated α-syn. The E3 ubiquitin ligase Listerin was found to promote K27-linked polyubiquitination of α-syn, directing it to the endosome for subsequent degradation. We showed that the deletion of the Listerin gene exacerbates the neurodegenerative progression in a mouse model of PD, whereas the overexpression of Listerin effectively mitigates disease progression in PD mice. Consequently, our study reveals a mechanism for α-syn degradation and identifies Listerin as a promising therapeutic target for the treatment of PD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


