质子选择性调节甲醇分子C─H键的不对称活化
IF 2
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
C─H键在温和条件下的活化是有机分子转化的关键步骤。然而,甲醇中C─H键的同时活化会导致过度氧化,从而降低甲醛生成的选择性。我们通过调节无水甲醇溶液中的质子浓度,在温和条件下实现了甲醇中C─H键的选择性活化,显著提高了甲醇的脱氢速率。此外,我们建立了相关的分子模型来确定不对称氧化的位点,为进一步研究C─H键的活化提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
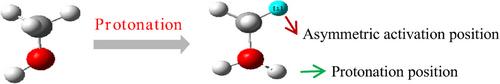
Protons Selectively Regulate the Asymmetric Activation of the C─H Bonds of Methanol Molecules
The activation of C─H bonds under mild conditions is a crucial step in the conversion of organic molecules. However, the simultaneous activation of C─H bonds in methanol can result in excessive oxidation, thereby diminishing the selectivity for formaldehyde production. We achieved selective activation of the C─H bonds in methanol under mild conditions by adjusting the proton concentration in an anhydrous methanol solution, which significantly enhanced the dehydrogenation rate of methanol. Additionally, we constructed related molecular models to identify the sites of asymmetric oxidation, providing new insights for further studies on C─H bond activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


