近环境条件下氨合成及硝酸盐原位CO2共还原制尿素
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
The Journal of Physical Chemistry Letters
Pub Date : 2025-02-03
DOI:10.1021/acs.jpclett.4c0329810.1021/acs.jpclett.4c03298
引用次数: 0
摘要
在这里,通过热活化过氧化氢(H2O2)在接近环境条件(~ 100°C)下证明了硝酸盐(NO3)转化为氨(NH3)高达~ 100 mM和原位级联CO2共还原为尿素(高达~ 1.36 mM)。通过吸附、1H核磁共振(NMR)分析还原产物和15N同位素标记硝酸盐(15NO3)实验,证实了NO3合成NH3。尿素合成的相关结果已通过吸收、1H NMR和高分辨率质谱(HR-MS)分析得到验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
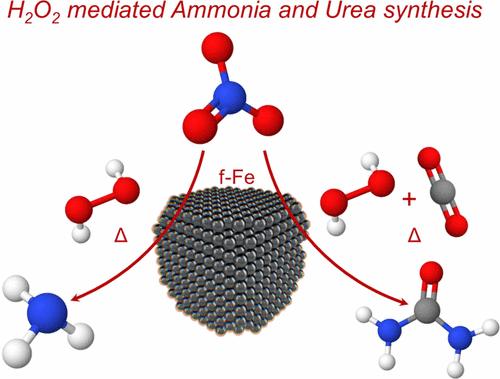
Near Ambient Condition Ammonia Synthesis and In-Situ CO2 Co-Reduction to Urea from Nitrate
Herein, conversion of nitrate (NO3̅) to ammonia (NH3) up to ∼100 mM and in-situ cascade CO2 co-reduction to urea (up to ∼1.36 mM) has been demonstrated by thermally activated hydrogen peroxide (H2O2) at near ambient conditions (∼100 °C). NH3 synthesis from NO3̅ has been confirmed using absorption, 1H nuclear magnetic resonance (NMR) analysis of the reduced product and 15N isotopic labeled nitratre (15NO3̅) experiement. The results associated with the urea synthesis have been verified using absorption, 1H NMR, and high resolution-mass spectrometry (HR-MS) analysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


