有机光氧化还原催化自由基远端取代芳酰氯的对选择性C-H烷基化
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
芳烃的位点选择性C(sp2) -H烷基化提供了在药物和农用化学品中普遍存在的片段。虽然邻选择性和间选择性烷基化反应已经很好地实现了,但准选择性烷基化反应的例子很少。目前可用的方法是根据金属的独特行为和特性设计的,以实现高的准选择性。本文通过有机光氧化还原催化自由基远端取代,证明了芳酰氯与烷基三氟硼酸盐和三烷基膦的准选择性C(sp2) -H烷基化反应。该反应通过对原位生成的芳基三烷基膦中间体进行烷基自由基的准选择性加成,然后进行氢位移。值得注意的是,对烷基化与羰基三烷基膦基转化为甲酰基有关,产生对烷基化苯甲醛衍生物。这种无金属位点选择性的C(sp2) -C (sp3)键形成允许流线型合成siponimod和SR-31747。本文章由计算机程序翻译,如有差异,请以英文原文为准。
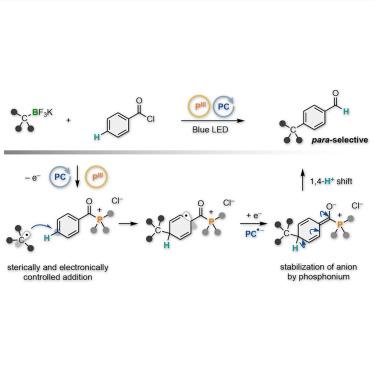
para-Selective C–H alkylation of aroyl chlorides through organic photoredox-catalyzed radical tele-substitution
The site-selective C(sp2)–H alkylation of arenes has provided ubiquitous fragments found in pharmaceutical drugs and agrochemicals. Although ortho- and meta-selective alkylation reactions have been achieved well, para-selective examples are scarce. The currently available methods have been designed on the unique behaviors and characteristics of metals to achieve high para-selectivity. Herein, we demonstrate the para-selective C(sp2)–H alkylation of aroyl chlorides with alkyltrifluoroborates and trialkylphosphine through an organic photoredox-catalyzed radical tele-substitution. The reaction proceeds through the para-selective addition of alkyl radicals to in-situ-generated aroyl trialkylphosphonium intermediates followed by a hydrogen shift. Notably, the para-alkylation is associated with the conversion of the carbonyl trialkylphosphonium group to the formyl group, producing para-alkylated benzaldehyde derivatives. This metal-free site-selective C(sp2)–C(sp3) bond formation allows for streamlined synthesis of siponimod and SR-31747.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


