光诱导自旋中心移位催化脱氧非活化1,2-二醇基序
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在此,我们报告了一种光诱导策略,该策略通过自旋中心移位(SCS)歧管实现了非活化脂肪族1,2-二醇衍生物的催化脱氧。根据烷基自由基中间体的性质,可以实现发散性反应。具体来说,仲醇产生α-脱氧酮,而在适当的自由基受体存在的情况下,伯醇可以促进C-C键的形成/自旋中心的移位,从而为SCS领域提供了一个未被认识的机会,可以重新利用现成的、未活化的1,2-二醇衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
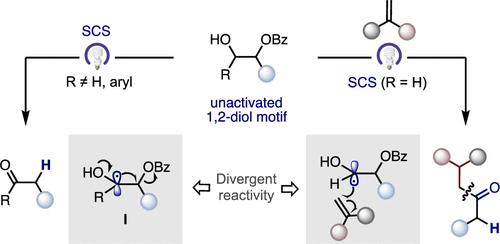
Catalytic Deoxygenation of Unactivated 1,2-Diol Motifs via Light-Induced Spin-Center Shift
Herein, we report a light-induced strategy that enables catalytic deoxygenation of unactivated aliphatic 1,2-diol derivatives via a spin-center shift (SCS) manifold. Divergent reactivity can be accomplished depending on the nature of the alkyl radical intermediate. Specifically, secondary alcohols result in α-deoxygenated ketones, whereas a tandem C–C bond-formation/spin-center shift can be promoted with primary alcohols instead in the presence of an appropriate radical acceptor, thus offering an unrecognized opportunity in the SCS arena for repurposing readily available, unactivated 1,2-diol derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


