铂电极与水/乙腈杂化电解质间双电层结构的研究
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
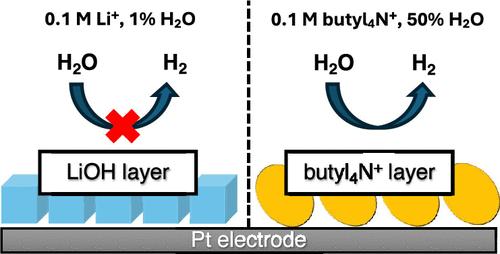
控制水在电催化界面上的反应活性是许多电催化反应的关键挑战。它的反应性可以通过改变混合水/有机电解质的组成来调节。为了推进这种方法,有必要了解电极/混合电解质界面的结构如何取决于电极电位。这种理解在很大程度上是缺乏的。本文中,我们利用表面增强红外吸收光谱(SEIRAS)探测了Pt电极与含有0.1 M LiClO4或(butyl4N)ClO4的乙腈/水混合物之间形成的界面。在Li+存在的情况下,随着电位的降低,晶体LiOH沉积在电极上的电解质具有低含水量(约1%重量),这是由于这种盐在乙氮中的溶解度低,阻断了析氢反应(HER)的活性位点。在丁基4n +存在下,表面疏水性增强,电势减小。值得注意的是,只有在高含水量的电解质中,丁基氮离子才会形成不可逆的物理吸附层。尽管形成了层,电极对HER仍保持活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating the Electric Double-Layer Structures between a Pt Electrode and Water/Acetonitrile Hybrid Electrolytes
Controlling the reactivity of water at electrocatalytic interfaces is a critical challenge in many electrocatalytic reactions. Its reactivity can be adjusted by altering the composition of hybrid aqueous/organic electrolytes. To advance this approach, it is essential to understand how the structure of the electrode/hybrid electrolyte interface depends upon the electrode potential. This understanding is largely lacking. Herein, using surface-enhanced infrared absorption spectroscopy (SEIRAS), we probed the interfaces formed between a Pt electrode and acetonitrile/water mixtures containing 0.1 M LiClO4 or (butyl4N)ClO4. In the presence of Li+ and with decreasing potential, crystalline LiOH deposits on the electrode in electrolytes with a low water content (∼1% by weight) due to the low solubility of this salt in acetonitrile, blocking the active sites of the hydrogen evolution reaction (HER). In the presence of butyl4N+, the surface becomes more hydrophobic with decreasing potential. Notably, butyl4N+ ions form an irreversibly physisorbed adlayer solely in electrolytes with a high water content. Despite the formation of the adlayer, the electrode remains active for the HER.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


