一种ph响应和富含胍的纳米佐剂有效地为癌症免疫治疗提供STING激动剂
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
作为干扰素基因刺激因子(STING)的天然激动剂,环二核苷酸(cdn)已被确定为有前景的免疫疗法,可引发针对肿瘤的有效免疫反应。然而,低稳定性、快速清除、细胞摄取不足和低效的细胞质定位严重阻碍了亲水和带负电的2 ',3 ' -环gmp - amp (cGAMP)的治疗效果。如何有效地将cGAMP传递到内质网(ER)以激活STING进行免疫启动仍然是一个挑战。在这里,我们报道了一种ph响应和富胍的STING纳米激动剂(nPGSA),用于细胞溶胶递送cGAMP。与游离cGAMP相比,nPGSA的细胞内化能力提高了37.4倍。ph敏感和胍功能设计有助于快速释放和内核体逃逸,从而实现cGAMP的精确ER靶向和STING敏化的33.9倍扩增。此外,通过调节肿瘤相关巨噬细胞(TAM)极化,nPGSA在黑色素瘤和神经母细胞瘤小鼠中引发了有效的抗原特异性细胞免疫反应和持续的肿瘤消退。我们的研究为cGAMP的递送提供了一个有希望的策略,并为癌症免疫治疗提供了cGAMP在调节肿瘤免疫微环境中的功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
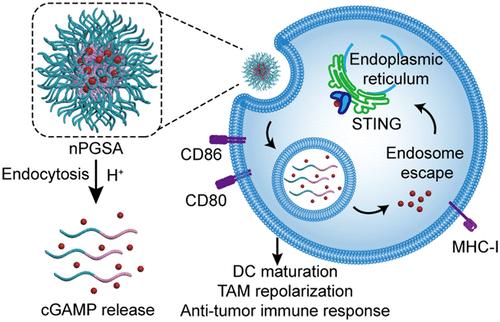
A pH-Responsive and Guanidinium-Rich Nanoadjuvant Efficiently Delivers STING Agonist for Cancer Immunotherapy
As natural agonists of the stimulator of interferon genes (STING), cyclic dinucleotides (CDNs) have been identified as promising immunotherapies that trigger a potent immune response against tumors. However, the low stability, rapid clearance, inadequate cellular uptake, and inefficient cytosol localization heavily hinder the therapeutic efficacy of the hydrophilic and negatively charged 2′, 3′-cyclic-GMP-AMP (cGAMP). How to efficiently deliver cGAMP into the endoplasmic reticulum (ER) to activate STING for immune priming remains challenging. Here, we report a pH-responsive and guanidinium-rich STING nanoagonist (nPGSA) for cytosol delivery of cGAMP. Compared with free cGAMP, nPGSA achieves up to a 37.4-fold enhancement of cellular internalization. The pH-sensitive and guanidinium-functional design facilitates quick release and endosome escape, thus enabling precise ER targeting of cGAMP and 33.9-fold amplification of STING sensibilization. Furthermore, through the modulation of tumor-associated macrophage (TAM) polarization, nPGSA elicits a potent antigen-specific cellular immune response and sustained tumor regression in melanoma- and neuroblastoma-bearing mice. Our study provides a promising strategy for the delivery of cGAMP, and it offers insights into the function of cGAMP in modulating the tumor immune microenvironment for cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


