通过碘试剂促进的分子内加氢反应合成非对映的氢苯并呋喃和氢萘并呋喃
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
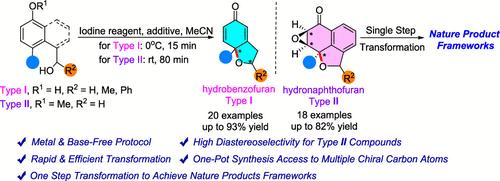
Synthesis of Diastereomeric Hydrobenzofurans and Hydronaphthofurans via an Iodine Reagent-Promoted Intramolecular Dearomatization Reaction
The first metal- and base-free construction of diastereomeric hydrobenzofurans and hydronaphthofurans, which were capable of further transformations to achieve natural product frameworks, was achieved by the intramolecular oxidized dearomatization of phenol or naphthol derivatives via the promotion of iodine reagents. Enantioselective products were obtained through chiral substrates or iodine catalysts. This step-economical protocol built multiple chiral centers with extensive tolerance of various substrates, which resulted in a potential molecular library for developing functional polycyclic scaffolds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


