醋酸烯丙酯单体的活级联炔复分解聚合及聚合后的Tsuji-Trost反应改性
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
本文报道了一种适合通过Tsuji-Trost反应进行聚合后改性的含丙烯酸酯单体的设计和合成。通过动力学研究和双嵌段共聚物的形成证明,该单体能够进行活级联炔复分解聚合。所得到的聚合物适用于高效的钯催化的Tsuji-Trost反应,使用一系列氮、氧和硫亲核试剂来提供结构精化。此外,原始和修饰的聚合物具有酸响应降解性和进一步的可调性,这取决于结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
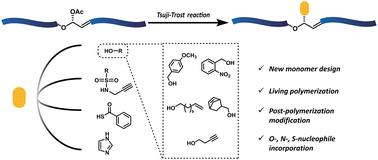
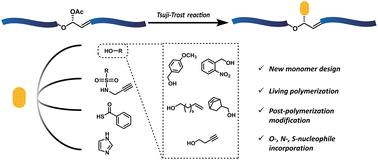
Living cascade enyne metathesis polymerization of an allylic acetate monomer and post-polymerization modification via a Tsuji–Trost reaction†
Here we report the design and synthesis of an allylic acetate-containing monomer suitable for post-polymerization modification through a Tsuji–Trost reaction. This monomer is capable of living cascade enyne metathesis polymerization, as evidenced by the kinetics studies as well as a diblock copolymer formation. The resultant polymers are amenable to efficient palladium-catalyzed Tsuji–Trost reactions using a range of nitrogen, oxygen, and sulfur nucleophiles to furnish structural elaboration. Additionally, the pristine and modified polymers possessed acid-responsive degradability and further tunability depending on the structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


