含硅酸盐添加剂的碱性溶液中针铁矿到绿铁的电化学还原途径
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在碱性溶液中高效低温铁电解是一种低成本、可持续、零碳排放的炼铁工艺。然而,电化学还原过程中Fe3O4的电化学惰性和寄生H2气体的形成阻碍了其实现,导致铁电解的能量效率较低。在这里,我们进一步探索了电化学还原针铁矿(FeOOH)的潜力,通过在碱性溶液中使用低浓度的硅酸盐添加剂来减轻Fe3O4的积累和H2的生成。电化学测量结合operando x射线衍射和x射线吸收光谱分析表明FeOOH→Fe3O4→Fe(OH)2→Fe还原途径。有趣的是,在NaOH/硅酸盐混合电解质中形成了一种结晶性差或无定形的Fe(OH)2相,这可能是由于硅酸盐对水和离子传输的抑制作用,这最终有助于改善Fe3O4的还原,这也得到了原子模拟的支持。这项工作证明了硅酸盐作为一种低成本和有效的电解质添加剂的潜力,可以通过电解改善室温下绿铁的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
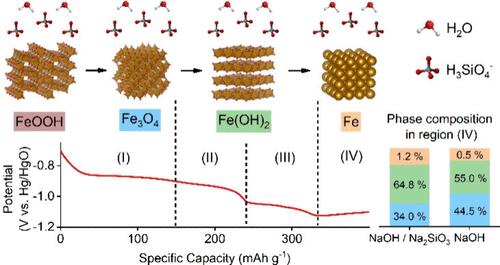
Electrochemical Reduction Pathways from Goethite to Green Iron in Alkaline Solution with Silicate Additive
Energy-efficient and low-temperature iron electrolysis in alkaline solutions is a low-cost and sustainable ironmaking process with zero-carbon emissions when renewable electrical sources are involved. However, its implementation is hindered by electrochemically inert Fe3O4 and parasitic H2 gas formation during the electrochemical reduction process, resulting in the low energy efficiency of iron electrolysis. Here, we further explore the potential of electrochemical reduction of goethite (FeOOH) by employing a low concentration of silicate additive in an alkaline solution to mitigate Fe3O4 accumulation and H2 generation. Electrochemical measurements coupled with operando X-ray diffraction and X-ray absorption spectroscopy suggested FeOOH → Fe3O4 → Fe(OH)2 → Fe reduction pathways. Interestingly, a poorly crystalline or amorphous Fe(OH)2 phase formed in the NaOH/silicate mixed electrolyte, possibly due to the inhibitive effect of silicate on water and ion transport, which eventually contributed to the improved reduction of Fe3O4, also supported by atomistic simulations. This work demonstrates the potential for silicate as a low-cost and effective electrolyte additive to improve room-temperature green iron formation via electrolysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


