复杂化学混合物的演化揭示了组合压缩和种群同步性
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
关于生命起源的许多悬而未决的问题都集中在复杂化学物种的产生上。过去的工作描述了可能导致生物分子的特定化学反应。在这里,我们建立了一个化学进化的实验模型来研究化学系统不断变化的一般过程。我们使用水作为化学反应物、产物和介质。我们利用水在接近环境温度下的振荡活性,在含有羧酸、胺、硫醇和羟基的有机分子混合物中引起接近平衡反应的棘轮。我们的系统(1)经历了不断的变化,过渡到新的化学空间,而不是在整个实验过程中收敛;(2)具有严格的化学选择的组合压缩;(3)显示分子居群的同步性。我们的研究结果表明,化学进化和选择可以在有机混合物中观察到,并可能最终适应于产生具有新结构和功能的广泛分子阵列。本文章由计算机程序翻译,如有差异,请以英文原文为准。

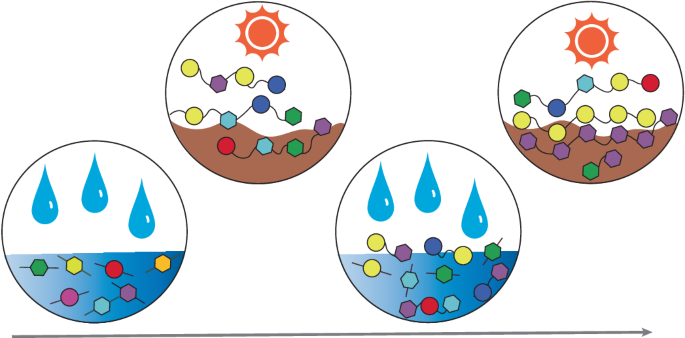
Evolution of complex chemical mixtures reveals combinatorial compression and population synchronicity
Many open questions about the origins of life are centred on the generation of complex chemical species. Past work has characterized specific chemical reactions that might lead to biological molecules. Here we establish an experimental model of chemical evolution to investigate general processes by which chemical systems continuously change. We used water as a chemical reactant, product and medium. We leveraged oscillating water activity at near-ambient temperatures to cause ratcheting of near-equilibrium reactions in mixtures of organic molecules containing carboxylic acids, amines, thiols and hydroxyl groups. Our system (1) undergoes continuous change with transitions to new chemical spaces while not converging throughout the experiment; (2) demonstrates combinatorial compression with stringent chemical selection; and (3) displays synchronicity of molecular populations. Our results suggest that chemical evolution and selection can be observed in organic mixtures and might ultimately be adapted to produce a broad array of molecules with novel structures and functions. Origins-of-life research has focused on specific chemical reactions that might lead to biological molecules. Now an experimental model of chemical evolution based on oscillating water activity has been established. This system undergoes continuous chemical change, and demonstrates combinatorial compression, stringent chemical selection and synchronicity of molecular populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


