用微乳液技术设计阿托品基隐形眼镜。
IF 3.7
3区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
摘要
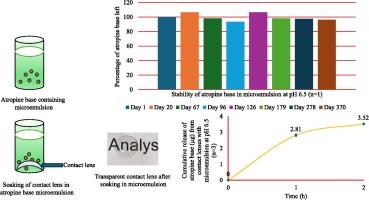
目的:近视是一个全球性的视力问题。阿托品滴眼液和控制近视的隐形眼镜可以帮助减缓近视的进展,但单独使用都是不够的。本研究工作是在微乳液系统中设计一种嵌入阿托品基的隐形眼镜。目的是提高阿托品碱的稳定性,促进其从晶状体中释放,防止使用阿托品滴眼液观察到的快速清除。方法:采用表面活性剂d - α -生育酚聚乙二醇1000琥珀酸酯(TPGS)、助表面活性剂聚乙二醇400 (peg400)、乳化剂Capmul MCM C8、阿托品碱和磷酸盐缓冲盐水(PBS)制备pH为7.4和pH为6.5的微乳。将微乳保存于室温(21℃),并在一年以上的时间内,利用反相高效液相色谱法(RPHPLC)定期检测微乳中阿托品碱的含量,以确定其稳定性。用zetasizer测量了配方的球状大小。微光隐形眼镜在阿托品碱微乳液中浸泡24 h,用RPHPLC测定阿托品碱装入隐形眼镜并在PBS中释放的量。采用ISO 10993-5标准测定阿托品碱型隐形眼镜的体外细胞毒性。结果:在pH为6.5 (ME为6.5)时,阿托品碱在微乳液中更稳定,降解率小于4%,而在pH为7.4 (ME为7.4)时,阿托品碱的降解率为10%。微乳液的粒径在17 ~ 21 nm之间。MiSight隐形眼镜从me6.5中吸收了4.25±1.67µg阿托品碱,大部分阿托品碱(3.52±0.03µg)在2 h内释放。然而,阿托品碱负载隐形眼镜的洗脱液对人角膜上皮细胞(HCECs)有毒性,24 h后细胞活力降低至5%以下。结论:微乳液稳定,隐形眼镜释放了足够量的阿托品碱,但需要进一步研究其毒性问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Designing a contact lens with atropine base using a microemulsion technique
Purpose
Myopia or near-sightedness is a global vision problem. Atropine eye drops and myopia-controlling contact lenses can help to slow down its progression, but neither is sufficient alone. The present research work was conducted to design a contact lens embedded with an atropine base within a microemulsion system. The goal was to improve the stability of atropine base and facilitate its release from the lens, preventing the rapid clearance observed with atropine eye drops.
Methods
Two microemulsions, one with a pH of 7.4 and the other with a pH of 6.5, were developed using the surfactant D-alpha-tocopherol polyethylene glycol 1000 succinate (TPGS), the co-surfactant polyethylene glycol 400 (PEG 400), the emulsifier Capmul MCM C8, atropine base, and phosphate-buffered saline (PBS). The microemulsions were kept at room temperature (21 °C) and the amount of the atropine base in microemulsions were checked periodically over one year using reverse-phase High Performance Liquid Chromatography (RPHPLC) to determine its stability. The globule size of the formulations was measured using a zetasizer. MiSight contact lenses were soaked in the atropine base microemulsion formulations for 24 h, and the amount of atropine base loaded into contact lenses and released in PBS was measured by a RPHPLC. ISO 10993-5 guidelines were used to measure the in vitro cytotoxicity of atropine base loaded contact lenses.
Results
The atropine base was more stable in the microemulsion at pH 6.5 (ME 6.5) with less than 4 % degradation, compared to a 10 % degradation at pH 7.4 (ME 7.4). The globule sizes of the microemulsions ranged between 17–21 nm. MiSight lenses absorbed 4.25 ± 1.67 µg atropine base from ME 6.5, with the majority of the atropine base (3.52 ± 0.03 µg) released within 2 h. However, elutes from atropine base loaded contact lenses were toxic to human corneal epithelial cells (HCECs), reducing cell viability to less than 5 % after 24 h.
Conclusions
While the microemulsions were stable and the contact lenses released sufficient amounts of atropine base, future studies are needed to address the toxicity issue.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Contact Lens & Anterior Eye
OPHTHALMOLOGY-
CiteScore
7.60
自引率
18.80%
发文量
198
审稿时长
55 days
期刊介绍:
Contact Lens & Anterior Eye is a research-based journal covering all aspects of contact lens theory and practice, including original articles on invention and innovations, as well as the regular features of: Case Reports; Literary Reviews; Editorials; Instrumentation and Techniques and Dates of Professional Meetings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


