由水和NFSI促进的邻羟基芳胺酮的溶剂依赖氟化环化:二氟化和单氟化2-羟基激素的可切换获取。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在无金属的条件下,通过环化反应将氟整合到杂环结构中是有机合成的一个有吸引力的方法。在此,我们描述了在无金属条件下由水和NFSI促进的邻羟基亚胺酮的溶剂依赖氟环化反应,以获得2,3取代的激素。二氟化和单氟化2-羟基激素可以在室温下在THF-H2O或EtOH-H2O体系中选择性地获得。本文章由计算机程序翻译,如有差异,请以英文原文为准。
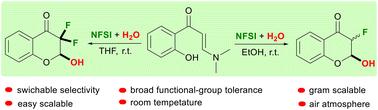
Solvent dependent fluorinative cyclizations of o-hydroxy-arylenaminones promoted by H2O and NFSI: switchable access to di- and monofluorinated 2-hydroxyl chromanones†
Integrating fluorine into heterocyclic structures via cyclization reactions under metal-free conditions is attractive for organic synthesis. Herein, we describe solvent dependent fluorinative cyclizations of o-hydroxyarylenaminones, which were promoted by H2O and NFSI under metal-free conditions, to furnish 2,3-substituted chromanones. Di- and monofluorinated 2-hydroxyl chromanones could be achieved selectively in a THF–H2O or EtOH–H2O system at ambient temperature under an air atmosphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


