表面工程纳米颗粒增强含血红素蛋白过氧化物酶活性
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
与纳米材料接触的酶通常会失去90%以上的活性;然而,一些纳米材料已经被证明可以增强酶的活性。然而,这些发现在很大程度上是观察性的,缺乏明确和可操作的设计原则。需要系统的研究来开发能够控制和调节酶活性的纳米材料。鉴于酶-纳米材料的相互作用是由它们的表面官能团介导的,我们假设工程纳米颗粒表面可以允许控制酶活性的调节。在这项研究中,我们使用肽功能化金纳米粒子(PGNPs)作为可编程平台来研究表面功能化如何影响酶的活性。通过改变多肽序列,我们研究了电荷、疏水性、多肽长度和结构对细胞色素C (Cyt C)过氧化物酶活性的影响。我们的结果表明,精心设计的配体可以显著提高酶活性,与游离酶相比,其活性超过10倍。分子动力学模拟提供了对这些发现的分子基础的见解,揭示了Cyt C在吸附时的首选取向以及酶与肽配体之间的关键相互作用模式,从而将实验结果与机制理解联系起来。此外,PGNPs被证明是一个多功能平台,可将其他含血红素的蛋白质(如乳酸过氧化物酶、血红蛋白和过氧化氢酶)的过氧化物酶活性分别提高13.4倍、3.9倍和4.2倍。这项研究强调了纳米颗粒表面工程在界面上以可调方式激活酶的潜力,为开发生物催化剂提供了一种有希望的替代蛋白质工程的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
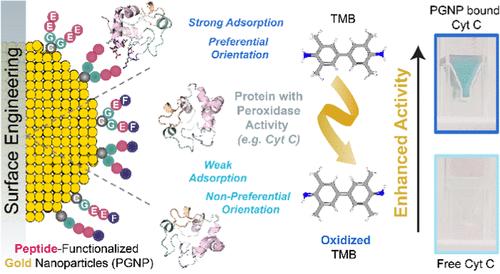
Surface-Engineered Nanoparticles Enhance the Peroxidase Activity of Heme-Containing Proteins
Enzymes interfaced with nanomaterials often lose more than 90% of their activity; yet, some nanomaterials have been shown to enhance enzyme activity. However, these findings are largely observational and lack clear and actionable design principles. Systematic studies are needed to develop nanomaterials that can control and tune enzyme activity. Given that enzyme–nanomaterial interactions are mediated by their surface functional groups, we hypothesized that engineering nanoparticle surfaces could allow for controlled tuning of the enzyme activity. In this study, we used peptide-functionalized gold nanoparticles (PGNPs) as a programmable platform to investigate how surface functionalization affects enzyme activity. By varying the peptide sequences, we examined the effects of charge, hydrophobicity, peptide length, and structure on the peroxidase activity of cytochrome C (Cyt C). Our results showed that carefully designed ligands can significantly enhance enzyme activity, exceeding 10-fold compared with the free enzyme. Molecular dynamics simulations provided insights into the molecular basis of these findings, revealing the preferred orientation of Cyt C upon adsorption and key interaction patterns between the enzyme and peptide ligands, thus bridging experimental results with a mechanistic understanding. Furthermore, PGNPs proved to be a versatile platform for boosting peroxidase activity of other heme-containing proteins such as lactoperoxidase, hemoglobin, and catalase by 13.4-, 3.9-, and 4.2-fold, respectively. This study highlights the potential of nanoparticle surface engineering to activate enzymes at interfaces in a tunable manner, offering a promising alternative to protein engineering for developing biocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


