ZIF-8作为可重复使用的磁性吸附剂,在三维石墨烯上修饰,用于高效去除废水中的孔雀石绿
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属有机框架,如ZIF-8,以其高染料吸附性能而闻名,但它们的实际应用受到从溶液中分离它们的困难的限制,使回收变得复杂。为了克服这一问题,本研究引入了一种三维磁性聚多巴胺修饰石墨烯气凝胶(MGA)作为ZIF-8的支撑,使耗尽的吸附剂易于分离和回收。MGA是以氧化石墨烯(GO)、多巴胺和氧化铁(Fe3O4)为原料合成的。然后通过原位浸渍技术将ZIF-8晶体加载到MGA衬底上,生产出一种新型的ZIF-8复合气凝胶(MZGA)。制备的MZGA具有472 m2/g的大比表面积,对孔雀石绿(MG)有良好的吸附能力。研究考察了pH(2 ~ 6)、初始染料浓度(20 ~ 1000mg /L)、温度(25、35、45℃)对MZGA吸附mg性能的影响。结果表明,在mg初始浓度为1000 mg/L、吸附剂剂量为10 mg、温度为25℃、pH为4的条件下,MZGA对mg的最大吸附量为6265.6 mg/g。吸附过程遵循Langmuir吸附等温线和拟二级动力学模型,表现出较强的单层化学吸附。详细的表面表征表明,MZGA去除MG的主要机制包括孔隙填充、静电吸引、氢键、Lewis酸碱相互作用和π-π相互作用。此外,MZGA表现出增强的磁性能(Ms = 39.4 emu/g),易于通过施加磁场去除耗尽的吸附剂。经过10次循环后,回收的MZGA仍能保持约91%的MG吸附量,表明其具有良好的可重复使用性。MZGA还测试了从实际水环境(包括海水、自来水和污水处理厂出水)中去除MG的能力,去除率超过98%。因此,MZGA作为一种去除废水中有机污染物的高效吸附剂显示出巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
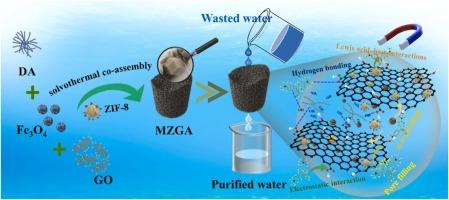
ZIF-8 decorated on three-dimensional graphene as reusable magnetic adsorbent for efficient removal of malachite green from wastewater
Metal-organic frameworks, such as ZIF-8, are well-known for their high dye adsorption performance, but their practical application is limited by difficulties in separating them from solutions, complicating recycling. To overcome this issue, this study introduces a three-dimensional magnetic polydopamine-modified graphene aerogel (MGA) as a support for ZIF-8, enabling easy separation and recycling of the exhausted adsorbent. MGA is synthesized using graphene oxide (GO), dopamine, and iron oxide (Fe3O4) as raw materials. ZIF-8 crystals are then loaded onto the MGA substrate via an in situ immersion technique, producing a novel ZIF-8 composite aerogel (MZGA). The resulting MZGA exhibits a large specific surface area of 472 m2/g and an excellent adsorption capacity for malachite green (MG). The study examines the effects of pH (ranging from 2 to 6), initial dye concentration (from 20 to 1000 mg/L), and temperature (25, 35, and 45 ℃) on the MG adsorption performance of MZGA. The results demonstrate that MZGA achieves a maximum adsorption capacity of 6265.6 mg/g for MG at an initial MG concentration of 1000 mg/L, an adsorbent dose of 10 mg, a temperature of 25 °C, and a pH of 4. The adsorption process follows the Langmuir adsorption isotherm and the pseudo-second-order kinetic model, suggesting strong monolayer chemisorption. Detailed surface characterization reveals that the primary mechanisms involved in MG removal by MZGA include pore filling, electrostatic attraction, hydrogen bonding, Lewis acid-base interactions, and π-π interactions. Additionally, MZGA exhibits enhanced magnetic properties (Ms = 39.4 emu/g), facilitating the easy removal of the exhausted adsorbent by applying a magnetic field. The recycled MZGA maintains approximately 91% of its initial MG adsorption capacity even after 10 cycles, demonstrating its excellent reusability. MZGA is also tested for the removal of MG from real aqueous environments, including seawater, tap water, and wastewater treatment plant effluent, achieving removal rates exceeding 98%. Thus, MZGA shows significant potential as an efficient adsorbent for removing organic pollutants from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


