联氨使酚类衍生物氢化脱氧获得芳烃
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氢脱氧(HDO)是将木质素及其衍生酚类化合物转化为高附加值芳香化学品和燃料的有效方法。利用分子氢来去除木质素衍生的酚类化合物中的羟基,使其在化学工业中具有吸引力。然而,这些工艺依赖于高压和昂贵的催化剂,在安全性、储氢和成本效益方面提出了挑战。这突出了在更容易获得的反应条件下对替代品的需求。在此,我们提出了一种以钯/碳为商业非均相催化剂,以肼为还原和形成腙的双重试剂的苯酚和萘酚的HDO方法。本文介绍了不同萘酚和酚类化合物的HDO的适用底物范围,包括药学上相关的分子,如扑热息痛。此外,高挑战性的类固醇衍生物,如β-雌二醇,已被氢脱氧。本文章由计算机程序翻译,如有差异,请以英文原文为准。
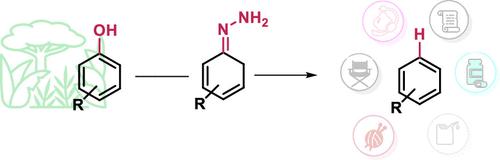
Accessing Arenes via the Hydrodeoxygenation of Phenolic Derivatives Enabled by Hydrazine
Hydrodeoxygenation (HDO) is an effective method for converting lignin and its derived phenolic compounds to value-added aromatic chemicals and fuels. Efforts to exploit molecular hydrogen have been made to remove the hydroxyl group in lignin-derived phenolic compounds to make them appealing for the chemical industry. However, these processes rely on high pressure and expensive catalysts, presenting challenges in terms of safety, hydrogen storage, and cost-effectiveness. This highlights the demand for alternatives under more accessible reaction conditions. Herein, we present a methodology for the HDO of phenols and naphthols using Pd/C as a commercial heterogeneous catalyst employing hydrazine as a dual reagent for reducing and hydrazone formation. This paper presents an applicable substrate scope for the HDO of different naphthols and phenols including pharmaceutically relevant molecules such as paracetamol. Additionally, highly challenging steroid derivatives, such as β-estradiol, have been hydrodeoxygenated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


