手性卟啉铱络合物催化烯烃的高度非对映选择性和非对映选择性环丙化反应
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
对手性卟啉-金属配合物(M = Co, Rh, Ru, Fe)催化烯烃不对称环丙烷化反应进行了广泛的研究。然而,相应的铱配合物很少被用于这一目的。本文报道了2,2,2-三氯乙基(TCE) α-芳基重氮乙酸酯1与烯烃2在催化量[Ir(Por *)(CO)Cl] (1.0 mol %)存在下的反应,以高收率和优异的对映选择性生成1,1,2-三取代环丙烷的单一非对映体。最近在我们实验室开发的一种β-轴向手性卟啉作为一种有效的支撑配体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
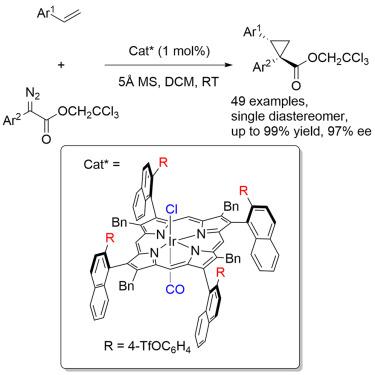
Highly diastereoselective and enantioselective cyclopropanation of alkenes catalyzed by a chiral iridium(III) porphyrin complex
Extensive research has been conducted on chiral porphyrin-metal complex (M = Co, Rh, Ru, Fe)-catalyzed asymmetric cyclopropanation of alkenes. However, the corresponding iridium complex has scarcely been exploited for this purpose. We report herein that the reaction of 2,2,2-trichloroethyl (TCE) α-aryldiazoacetates 1 with alkenes 2 in the presence of a catalytic amount of [Ir(Por∗)(CO)Cl] (1.0 mol %) yields a single diastereomer of 1,1,2-trisubstituted cyclopropanes in high yields with excellent enantioselectivities. A β-axially chiral porphyrin, developed recently in our laboratory, serves as an efficient supporting ligand.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


