酮类的还原偶联:铝基阴离子和没食子基阴离子反应性的对比
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
报道了一种新的没食子酸钾体系K[Ga(NON)] ([NON]2 - = [O(SiMe2NDipp)2]2 -, Dipp = 2,6- ipr2c6h3)的合成,并对其对多种酮的反应性进行了研究。没食子酸钾最初被分离为接触二聚体对(CDP) [K{Ga(NON)}]2,但在加入配位多齿配体后可转化为单体离子对(MIP) (NON)Ga - K(TMEDA)2或分离离子对(SIP) [K(222-crypt)][Ga(NON)]。CDP与每个镓中心两个等效的二苯甲酮或9-芴酮反应生成均偶联的pinacolate产物。然而,与苯乙酮的反应最初生成1,3-苯基-1-酸镓,其C-C与第二等价物苯乙酮偶联形成前所未有的1,3-二苯基丁烷-1,3-二酸镓。这种反应活性与类似的铝基钾CDP [K{Al(NON)}]2进行了对比,在相同的反应条件下,观察到pinacolate产物的优先形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
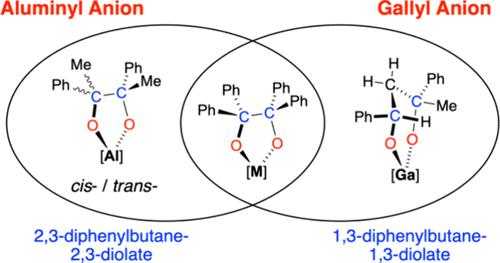
Reductive Coupling of Ketones: Contrasting the Reactivity of an Aluminyl and a Gallyl Anion
The synthesis of a new potassium gallyl system, K[Ga(NON)] ([NON]2– = [O(SiMe2NDipp)2]2–, Dipp = 2,6-iPr2C6H3), has been reported, and its reactivity toward a variety of ketones has been studied. The potassium gallyl is initially isolated as the contacted dimeric pair (CDP) [K{Ga(NON)}]2 but can be converted to the monomeric ion pair (MIP) (NON)Ga–K(TMEDA)2 or the separated ion pair (SIP) [K(222-crypt)][Ga(NON)] upon addition of coordinating polydentate ligands. The reaction of the CDP with two equivalents of benzophenone or 9-fluorenone per gallium center generates homocoupled pinacolate products. However, the reaction with acetophenone initially forms a gallium 1,3-phenylethene-1-olate, which C–C couples to a second equivalent of acetophenone to form an unprecedented gallium 1,3-diphenylbutane-1,3-diolate. This reactivity is contrasted with the analogous potassium aluminyl CDP [K{Al(NON)}]2, where preferential formation of the pinacolate product is observed under the same reaction conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


