Pd/ c催化硝基芳烃与甲醇转移加氢环氧化物串联合成β-氨基醇
IF 4.3
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
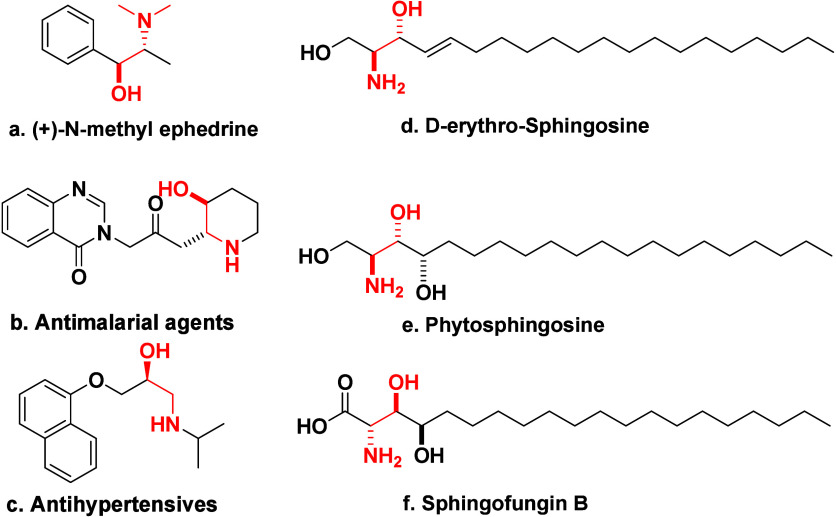
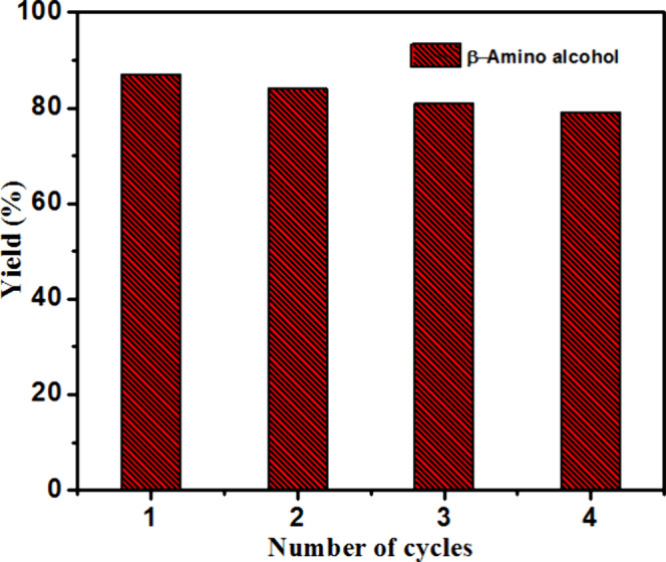
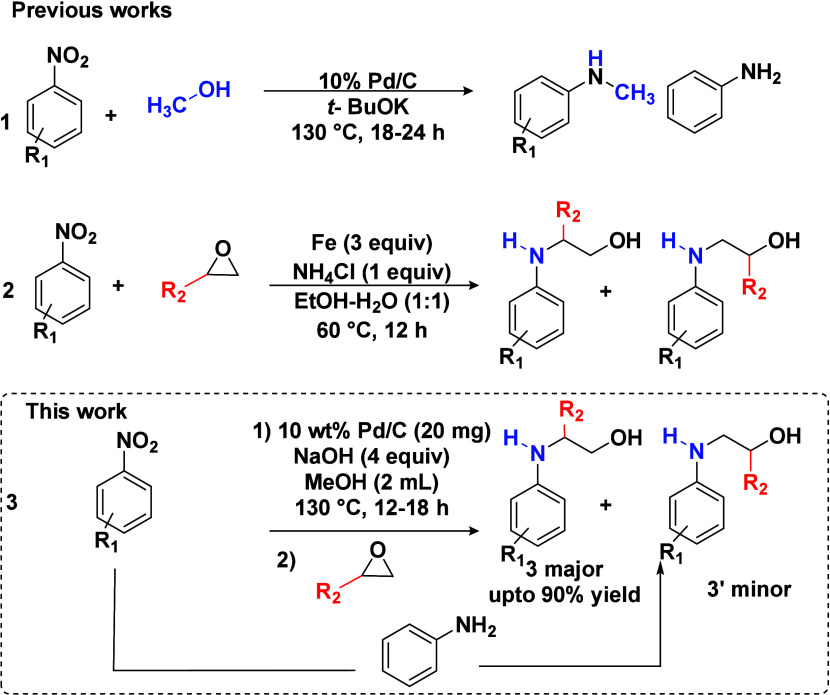
以市售Pd/C为催化剂,由硝基芳烃和1,2-环氧化物合成β-氨基醇。硝基芳烃通过环氧化物开环和甲醇在Pd/C催化剂上的转移加氢转化为胺,生成β-氨基醇。这种方法从硝基苯中原位生成苯胺,使其成为传统β-氨基醇合成方法的可行替代方法。该技术具有过滤回收催化剂容易、不需要中间分离等优点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pd/C-Catalyzed Tandem Synthesis of β-Amino Alcohol Using Epoxide through Transfer Hydrogenation of Nitroarene with Methanol.
Using commercially available Pd/C as a catalyst, β-amino alcohols are synthesized from nitroarenes and 1,2-epoxides. Nitroarenes are transformed to amines through ring opening of epoxides and transfer hydrogenation of methanol over the Pd/C catalyst, producing β-amino alcohols. This approach generates aniline in situ from nitrobenzene, making it a viable alternative to traditional β-amino alcohol synthesis procedures. This technique has advantages, including easy recovery of catalysts through filtration and elimination of the requirement for intermediate separation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


