硝基胺和碳二亚胺的1,3偶极环加成法合成二/三氟甲基双(1,2,4-三唑啉)螺旋烷和1,2,4-三唑。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种通过硝基胺和碳二酰亚胺的1,3偶极环加成反应合成二/三氟甲基双(1,2,4-三唑啉)螺旋烷和1,2,4-三唑的高效方法。各种n -芳基二/三氟甲基乙酰肼酰溴在反应中具有良好的耐受性,提供了良好的区域选择性和产率的目标产物。该方法操作简单,效率高,反应条件温和,可快速合成富氮氟烷基化复杂螺环化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
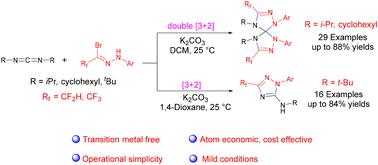
Synthesis of di/trifluoromethyl bis(1,2,4-triazoline)spiranes and 1,2,4-triazoles via 1,3-dipolar cycloaddition of nitrilimines and carbodiimides†
An efficient strategy for constructing di/trifluoromethyl bis(1,2,4-triazoline)spiranes and 1,2,4-triazoles via 1,3-dipolar cycloaddition reaction of nitrilimines and carbodiimides was developed. Various N-aryl di/trifluoromethyl acetohydrazonoyl bromides are well tolerated in the reaction, providing the target products in good regioselectivity and yields. This method features simple operation, high efficiency and mild reaction conditions for the rapid synthesis of complex fluoroalkylated nitrogen-rich spirocyclic compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


