Cu-ZnO催化马来酸酐直接加氢制1,4-丁二醇反应动力学研究
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
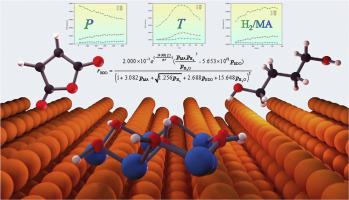
1,4-丁二醇是生产生物可降解塑料(如PBS和PBAT)的重要单体,也可用于合成γ-丁内酯和四氢呋喃等衍生物。在Cu-ZnO上直接加氢马来酸酐制1,4-丁二醇的工艺具有工艺流程短、反应条件温和、催化剂成本低、可联产多种产物等优点,是一种很有前途的生产方法。然而,目前尚不清楚马来酸酐在Cu-ZnO上直接加氢生成1,4-丁二醇的反应动力学方程和最佳条件,这阻碍了该生产工艺的发展。本研究在密度泛函理论的基础上,利用活性评价实验和动力学蒙特卡罗模拟研究了马来酸酐在Cu211表面和Cu211- zno界面上直接加氢的反应动力学。通过马来酸酐直接加氢活性评价实验,考察了温度、压力、氢酐比对Cu-ZnO催化剂上产物生成的影响,确定了1,4-丁二醇的最佳反应条件为200℃、2 MPa、氢酐比150。利用动力学蒙特卡罗方法,计算了在Cu211表面上马来酸酐直接加氢生成γ-丁内酯和在Cu211- zno界面上生成γ-丁内酯、1,4-丁二醇和四氢呋喃的反应过程。得到了马来酸酐生成γ-丁内酯、1,4-丁二醇和四氢呋喃的最佳反应条件。最后,拟合并推导了马来酸酐直接加氢制不同产物的本征反应动力学方程。所得到的本征反应动力学方程可以在考虑扩散影响后应用于实际反应器的设计或工艺开发中,也可以直接用于扩散效应不显著的研究中。这为在Cu-ZnO上直接加氢马来酸酐制1,4-丁二醇的生产工艺的构建提供了设计基础。希望本研究能为理解顺丁二酸酐直接加氢工艺及生物降解塑料产业的发展提供见解和指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of the reaction kinetics for the direct hydrogenation of maleic anhydride to 1,4-butanediol over Cu-ZnO catalyst
1,4-butanediol serves as a crucial monomeric building block for the production of biodegradable plastics such as PBS and PBAT, and it can also be utilized for the synthesis of derivatives like γ-butyrolactone and tetrahydrofuran. The technology of direct hydrogenation of maleic anhydride to 1,4-butanediol over Cu-ZnO has emerged as a promising production method due to its advantages of a short process flow, mild reaction conditions, cost-effective catalysts, and the capability to co-produce various products. However, at the current stage, the reaction kinetic equations and optimal conditions for 1,4-butanediol formation via direct hydrogenation of maleic anhydride over Cu-ZnO remain unclear, which hinders the development of this production process.
In this study, based on the findings of density functional theory, this study investigated the reaction kinetics of direct hydrogenation of maleic anhydride on Cu211 surfaces and at Cu211-ZnO interfaces using activity evaluation experiments and kinetic Monte Carlo simulations. Through activity evaluation experiments of direct hydrogenation of maleic anhydride, this study examined the effects of temperature, pressure, and hydrogen-to-anhydride ratio on product formation over Cu-ZnO catalysts and identified the optimal reaction conditions for 1,4-butanediol production as 200 °C, 2 MPa, and a hydrogen-to-anhydride ratio of 150. Utilizing the kinetic Monte Carlo method, this study calculated the reaction processes for the direct hydrogenation of maleic anhydride to γ-butyrolactone on Cu211 surfaces and to γ-butyrolactone, 1,4-butanediol, and tetrahydrofuran at Cu211-ZnO interfaces. The optimal reaction conditions for the formation of maleic anhydride to γ-butyrolactone, 1,4-butanediol, and tetrahydrofuran were obtained, respectively. Finally, intrinsic reaction kinetic equations for the direct hydrogenation of maleic anhydride to different products were fitted and derived.
The obtained intrinsic reaction kinetic equations can be applied in the design of actual reactors or process development after considering the influence of diffusion or directly used in studies where diffusion effects are insignificant. This provides a design foundation for the construction of production processes for direct hydrogenation of maleic anhydride to 1,4-butanediol over Cu-ZnO. It is hoped that this study can offer insights and guidance for the understanding of the direct hydrogenation process of maleic anhydride and the development of the biodegradable plastics industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


