硝基四唑紫碘在酸性条件下对低碳钢的耐蚀性的实验与理论研究
IF 7.3
2区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
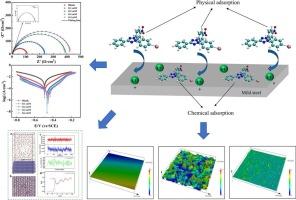
碘硝基紫四氮唑(INT)在生物和医学领域有着广泛的应用。含氮杂环具有良好的缓蚀效果。因此,INT在腐蚀控制领域显示出作为缓蚀剂的潜力。通过实验研究和计算分析,探讨了四氮唑衍生物在防腐领域的适用性。采用电化学技术研究了INT在1 M HCl溶液中对低碳钢的防腐效果。电化学测试表明,在298.15 K室温下,0.4 mM浓度的缓蚀剂INT的缓蚀效果最好,缓蚀效率可达98.38%。此外,还研究了KI和KCl的加入对缓蚀性能的影响。用吸附曲线描述吸附行为,发现INT符合Langmuir吸附等温线。扫描电镜(SEM)、激光扫描共聚焦显微镜(LSCM)、接触角(CA)和x射线光电子能谱(XPS)为INT对金属衬底的保护作用提供了额外的证据。通过分子动力学模拟和量子化学计算来深入了解吸附机理。发现INT分子通过平行吸附最大程度地覆盖在低碳钢表面,并与质谱表面形成π配合物。总的来说,实验结果和理论结果是一致的,表明INT对腐蚀有显著的抑制作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Corrosion resistance of mild steel under acidic conditions using iodine nitrotetrazolium violet: Experimental and theoretical research
Iodine nitrotetrazolium violet (INT) is commonly used in the fields of biology and medicine. Nitrogen-containing heterocycles typically exhibit good corrosion inhibition effects. Therefore, INT exhibits potential as a corrosion inhibitor in the realm of corrosion control. The applicability of tetrazolium derivatives INT in the area of anti-corrosion was explored through experimental investigations and computational analyses. In this research, electrochemistry technique was applied to investigate the corrosion prevention impact of INT on mild steel in 1 M HCl. Electrochemical testing indicated that the inhibitor INT with a concentration of 0.4 mM had the best corrosion inhibition effect at room temperature of 298.15 K, with an efficiency of up to 98.38 %. In addition, the effect of the addition of KI or KCl on the corrosion inhibition properties was also investigated. Adsorption curves were used to describe the adsorption behaviour and INT was found to conform to the Langmuir adsorption isotherm. Scanning electron microscopy (SEM), laser scanning confocal microscopy (LSCM), contact angle (CA) and X-ray photoelectron spectroscopy (XPS) provided additional evidence of the protective effect of INT on the metal substrate. Molecular dynamics simulations and quantum chemical calculations were carried out to gain insight into the adsorption mechanism. It was found that INT molecules covered the surface of mild steel to the maximum extent through parallel adsorption and form π complexes with the MS surface. Overall, there is consistency between the experimental and theoretical findings, revealing that INT has a notable inhibitory effect on corrosion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Progress in Organic Coatings
工程技术-材料科学:膜
CiteScore
11.40
自引率
15.20%
发文量
577
审稿时长
48 days
期刊介绍:
The aim of this international journal is to analyse and publicise the progress and current state of knowledge in the field of organic coatings and related materials. The Editors and the Editorial Board members will solicit both review and research papers from academic and industrial scientists who are actively engaged in research and development or, in the case of review papers, have extensive experience in the subject to be reviewed. Unsolicited manuscripts will be accepted if they meet the journal''s requirements. The journal publishes papers dealing with such subjects as:
• Chemical, physical and technological properties of organic coatings and related materials
• Problems and methods of preparation, manufacture and application of these materials
• Performance, testing and analysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


