疾病相关变异的功能分析阐明NLRP3激活和抑制的机制
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
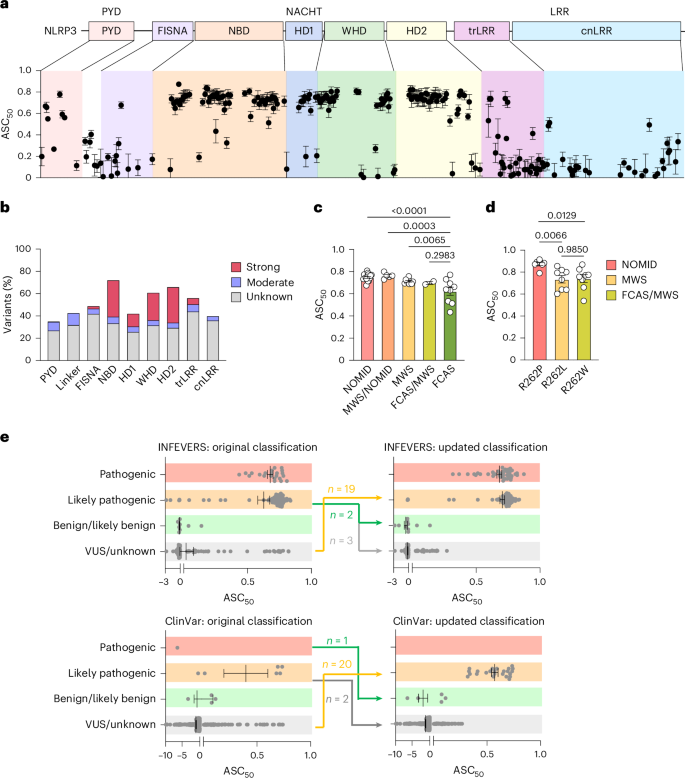
NLRP3炎性小体是一种多蛋白复合物,介导caspase-1激活和促炎细胞因子的释放,包括白细胞介素(IL)-1β和IL-18。编码NLRP3(也称为crypyrin)的基因的功能获得变异导致组成炎性体激活和过量的IL-1β产生在crypyrin相关周期性综合征(CAPS)中。在这里,我们介绍了来自国际infvers登记处和ClinVar数据库的534种NLRP3变异的功能筛选和自动分析。该资源捕获了NLRP3变异对ASC斑点自发形成的影响,在低温下,炎症小体刺激后,使用特异性NLRP3抑制剂MCC950。最值得注意的是,我们的分析促进了对infervers中NLRP3变异的最新分类。结构分析表明CAPS变体激活NLRP3的多种机制,包括增强ATP结合、稳定活性NLRP3构象、破坏非活性NLRP3复合物的稳定以及促进pyrin结构域的低聚化。此外,我们发现致病变异可以使NLRP3的激活对尼日利亚菌素和低温暴露产生超敏反应。我们还发现,大多数caps相关的NLRP3变异可以被MCC950抑制;然而,影响抑制剂结合位点附近螺旋的脯氨酸变化的NLRP3变体对MCC950具有抗性,pyrin结构域的变体也是如此,可能直接触发ASC pyrin结构域的激活。我们的研究结果可以帮助对NLRP3抑制剂临床试验的CAPS群体进行分层,我们的自动化方法可以应用于具有不同激活机制的分子,以及世界各地有兴趣向资源中添加新的功能验证的NLRP3变体的实验室。总的来说,我们的研究为CAPS患者提供了更好的诊断,对NLRP3激活的机制有了深入的了解,并为未来靶向治疗的应用提供了患者分层。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Mechanisms of NLRP3 activation and inhibition elucidated by functional analysis of disease-associated variants
The NLRP3 inflammasome is a multiprotein complex that mediates caspase-1 activation and the release of proinflammatory cytokines, including interleukin (IL)-1β and IL-18. Gain-of-function variants in the gene encoding NLRP3 (also called cryopyrin) lead to constitutive inflammasome activation and excessive IL-1β production in cryopyrin-associated periodic syndromes (CAPS). Here we present functional screening and automated analysis of 534 NLRP3 variants from the international INFEVERS registry and the ClinVar database. This resource captures the effect of NLRP3 variants on ASC speck formation spontaneously, at low temperature, after inflammasome stimulation and with the specific NLRP3 inhibitor MCC950. Most notably, our analysis facilitated the updated classification of NLRP3 variants in INFEVERS. Structural analysis suggested multiple mechanisms by which CAPS variants activate NLRP3, including enhanced ATP binding, stabilizing the active NLRP3 conformation, destabilizing the inactive NLRP3 complex and promoting oligomerization of the pyrin domain. Furthermore, we identified pathogenic variants that can hypersensitize the activation of NLRP3 in response to nigericin and cold temperature exposure. We also found that most CAPS-related NLRP3 variants can be inhibited by MCC950; however, NLRP3 variants with changes to proline affecting helices near the inhibitor binding site are resistant to MCC950, as are variants in the pyrin domain, which likely trigger activation directly with the pyrin domain of ASC. Our findings could help stratify the CAPS population for NLRP3 inhibitor clinical trials and our automated methodologies can be implemented for molecules with a different mechanism of activation and in laboratories worldwide that are interested in adding new functionally validated NLRP3 variants to the resource. Overall, our study provides improved diagnosis for patients with CAPS, mechanistic insight into the activation of NLRP3 and stratification of patients for the future application of targeted therapeutics. Gain-of-function variants in the gene encoding NLRP3 lead to constitutive inflammasome activation and excessive IL-1β production. In this resource, authors perform functional screening of clinically relevant NLRP3 variants. Structural analysis suggested multiple mechanisms by which variants activate NLRP3 and the identification of pathogenic variants that can sensitize the activation of NLRP3 in response to nigericin and cold temperature exposure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


