短可扩展的路线到双吗啡啉螺缩醛和恶西泮类似物:有用的3d支架的化合物库组装
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
含氮杂环的sp3-Rich分子支架是构建化合物筛选文库和药物发现的重要起点。在此,我们报告了一个四步合成构象明确的富含sp3的支架,其中包含嵌入在螺缩醛框架中的两个啉环。该合成涉及2-氯甲基取代的啉的中间体,由环氧氯丙烷和容易获得的β-氨基醇获得。碱介导的脱氢氯化反应产生一个外环烯醇醚,在此基础上分两步构造第二个啉环。支架合成收率高,可大规模进行。该方法允许将一个或两个啉环替换为1,4-恶西泮,并生成6,7-和7,7-螺缩醛类似物,这在药物发现中几乎没有被探索过。可以制备取代的6,6-体系,并且在某些情况下,通过酸介导的异构化以高非对映选择性递送支架。在6,6-和6,7-螺缩醛支架中嵌入的两种胺官能团依次被功能化,以提供多种物理化合物库。这些文库化合物与美国食品和药物管理局批准临床应用的小分子药物占据相似的化学空间,但结构不同,因此可能作用于新的靶点,代表了药物发现的有吸引力的起始材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
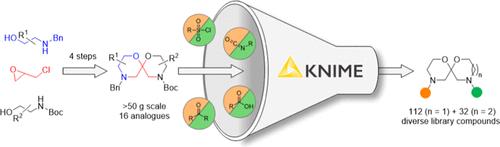
Short Scalable Route to Bis-morpholine Spiroacetals and Oxazepane Analogues: Useful 3D-Scaffolds for Compound Library Assembly
sp3-Rich molecular scaffolds incorporating nitrogen heterocycles represent important starting points for assembling compound screening libraries and drug discovery. Herein, we report a four-step synthesis of a conformationally well-defined sp3-rich scaffold incorporating two morpholine rings embedded within a spiroacetal framework. The synthesis involves the intermediacy of a 2-chloromethyl-substituted morpholine, accessed from epichlorohydrin and readily available β-aminoalcohols. Base-mediated dehydrochlorination affords an exocyclic enol ether, from which the second morpholine ring is constructed in two steps. Scaffold synthesis is high-yielding and can be performed on a large scale. The methodology allows ready substitution of one–or both– of the morpholine rings for 1,4-oxazepanes and the generation of 6,7- and 7,7-spiroacetal analogues, which are virtually unexplored in drug discovery. Substituted 6,6-systems can be prepared and, in some instances, undergo acid-mediated anomerization to deliver the scaffolds in high diastereoselectivity. The two amine functionalities embedded in the 6,6- and 6,7-spiroacetal scaffolds were sequentially functionalized to provide a diverse physical compound library. These library compounds occupy a similar chemical space to small-molecule drugs that have been approved for clinical application by the Food and Drug Administration yet are structurally dissimilar and may therefore act upon novel targets, representing attractive starting materials for drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


