基于快速响应吸附剂辅助智能模型的含铬废水高效处理
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
含有铬离子(Cr(VI))的废水具有重大的环境污染风险,并受到严格的监管。然而,由于工业生产的波动,这些废水中的Cr(VI)浓度变化很大。本研究在废水处理过程中采用快速响应吸附剂,处理速度显著提高。该吸附剂具有无定形结构,表面存在-OH和Zr-OH基团。在吸附过程中,吸附剂表面带正电荷,对Cr(VI)阴离子具有优异的吸附性能。吸附量可达97.9 mg/g。密度泛函理论(DFT)计算证实了负吸附能。Langmuir等温线模型和拟二级动力学表明,吸附过程是一个以化学吸附为主的单层吸附过程。6.78 kJ/mol的低活化能表明该吸附剂对Cr(VI)的响应速度快。该吸附剂在30 min内对模拟和真实含Cr(VI)废水均有较好的去除效果,且铬浓度达到排放标准。采用两隐层人工神经网络(ANN)模型对吸附结果进行了有效的分析和预测,R2为0.988。溶液放大实验进一步证明了该吸附剂在工业应用中的潜力。所制备的锆基吸附剂性能优良,具有良好的工业化应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。

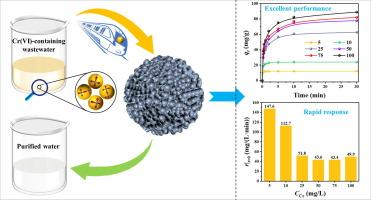
Efficient treatment of chromium-containing wastewater based on auxiliary intelligent model with rapid-response adsorbents
Wastewater containing chromium ions (Cr(VI)) presents substantial environmental pollution risks and is governed by stringent regulations. However, due to fluctuations in industrial production, the Cr(VI) concentrations in such wastewater vary significantly. This study employs rapid-response adsorbents in the wastewater treatment process, leading to a remarkable improvement in treatment speed. The adsorbent features an amorphous structure with –OH and Zr-OH groups present on its surface. During the adsorption process, the adsorbent surface showed a positive charge, enabling excellent adsorption performance for the Cr(VI) anion. The adsorption capacity reaches up to 97.9 mg/g. The Density Functional Theory (DFT) calculations verify negative adsorption energies. The Langmuir isotherm modeling and pseudo-second-order kinetic suggest that the adsorption process is a monolayer one, predominantly governed by chemisorption. The low activation energy of 6.78 kJ/mol indicates the rapid responsiveness of the adsorbent to Cr(VI). The adsorbent exhibits excellent performance in removing Cr(VI) from both simulated and real Cr(VI)-containing wastewater within 30 min, and the chromium concentration meets the emission standards. The adsorption results were effectively analyzed and predicted using an artificial neural network (ANN) model with two hidden layers, achieving an R2 of 0.988. The solution scale-up experiments further demonstrate the potential of this adsorbent for industrial applications. The zirconium-based adsorbents investigated in this study display excellent performance and hold promising prospects for industrialization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


