癌胎重编程驱动wnt依赖性结直肠癌的表型可塑性
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
靶向癌症干细胞(CSCs)对于有效的癌症治疗至关重要,但wnt驱动的结直肠癌(CRC)中LGR5+ CSC耗竭的耐药机制尚不清楚。在本研究中,我们揭示了突变肠干细胞(SCs)偏离其规范身份,穿越动态表型谱。这种增强的可塑性是由癌胎(OnF)重编程启动的,由YAP和AP-1驱动,随后AP-1过度激活促进谱系不忠。类视黄嘌呤X受体在大肠腺瘤性息肉病(APC)功能丧失后,作为OnF重编程及其解除管制的守门者,建立了由YAP和AP-1维持的OnF“记忆”。值得注意的是,OnF和LGR5+状态的临床意义受到其功能冗余的限制。虽然典型的LGR5+状态对FOLFIRI方案敏感,但活跃的OnF程序与耐药性相关,支持其在驱动耐药状态中的作用。结合当前的护理标准,针对这一计划是实现有效和持久的结直肠癌治疗的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。

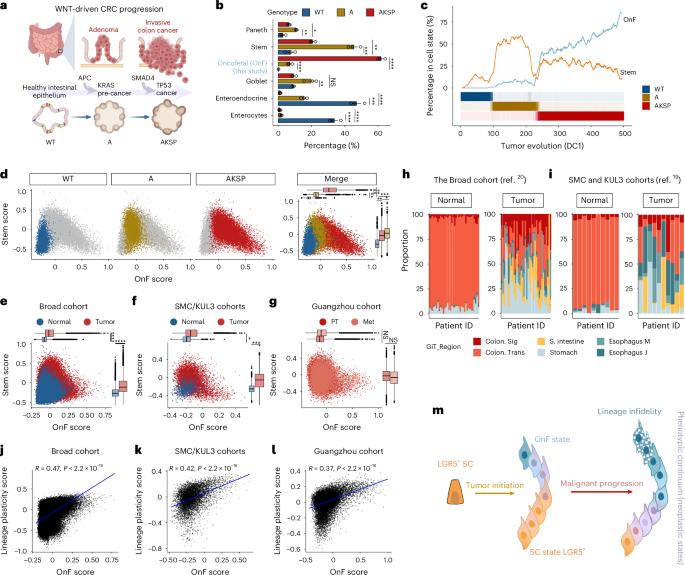
Oncofetal reprogramming drives phenotypic plasticity in WNT-dependent colorectal cancer
Targeting cancer stem cells (CSCs) is crucial for effective cancer treatment, yet resistance mechanisms to LGR5+ CSC depletion in WNT-driven colorectal cancer (CRC) remain elusive. In the present study, we revealed that mutant intestinal stem cells (SCs) depart from their canonical identity, traversing a dynamic phenotypic spectrum. This enhanced plasticity is initiated by oncofetal (OnF) reprogramming, driven by YAP and AP-1, with subsequent AP-1 hyperactivation promoting lineage infidelity. The retinoid X receptor serves as a gatekeeper of OnF reprogramming and its deregulation after adenomatous polyposis coli (APC) loss of function establishes an OnF ‘memory’ sustained by YAP and AP-1. Notably, the clinical significance of OnF and LGR5+ states in isolation is constrained by their functional redundancy. Although the canonical LGR5+ state is sensitive to the FOLFIRI regimen, an active OnF program correlates with resistance, supporting its role in driving drug-tolerant states. Targeting this program in combination with the current standard of care is pivotal for achieving effective and durable CRC treatment. Oncofetal (OnF) reprogramming, driven by YAP and AP-1, induces phenotypic plasticity and therapy resistance in WNT-dependent colorectal cancer (CRC). Targeting the OnF state in combination with chemotherapy substantially attenuates tumor growth in mouse models and patient-derived CRC tumoroids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


