乳酸通过表观遗传调控调控肿瘤的干性和可塑性
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肿瘤是由调节干细胞更新和分化的基因突变驱动的不受控制的细胞增殖引起的。然而,肠道肿瘤保留了一些等级组织,维持了癌症干细胞(CSCs)和癌症分化细胞(cdc)。这种异质性,加上细胞可塑性,使cdc恢复为csc,有助于治疗抵抗和复发。利用人类肿瘤类器官的基因编码荧光报告,结合我们基于机器学习的细胞跟踪器CellPhenTracker,我们同时追踪了肿瘤类器官发育过程中的细胞类型特征、代谢变化,并重建了细胞谱系轨迹。我们的研究结果揭示了CSCs和cdc中不同的代谢表型。我们发现乳酸调节肿瘤动力学,抑制CSC分化并诱导去分化进入增殖CSC状态。从机制上讲,乳酸增加组蛋白乙酰化,在表观遗传上激活MYC。鉴于乳酸对MYC的调控依赖于含溴结构域蛋白4 (BRD4),靶向肿瘤代谢和BRD4抑制剂成为预防肿瘤复发的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
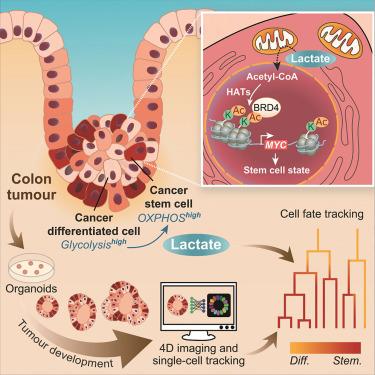
Lactate controls cancer stemness and plasticity through epigenetic regulation
Tumors arise from uncontrolled cell proliferation driven by mutations in genes that regulate stem cell renewal and differentiation. Intestinal tumors, however, retain some hierarchical organization, maintaining both cancer stem cells (CSCs) and cancer differentiated cells (CDCs). This heterogeneity, coupled with cellular plasticity enabling CDCs to revert to CSCs, contributes to therapy resistance and relapse. Using genetically encoded fluorescent reporters in human tumor organoids, combined with our machine-learning-based cell tracker, CellPhenTracker, we simultaneously traced cell-type specification, metabolic changes, and reconstructed cell lineage trajectories during tumor organoid development. Our findings reveal distinctive metabolic phenotypes in CSCs and CDCs. We find that lactate regulates tumor dynamics, suppressing CSC differentiation and inducing dedifferentiation into a proliferative CSC state. Mechanistically, lactate increases histone acetylation, epigenetically activating MYC. Given that lactate’s regulation of MYC depends on the bromodomain-containing protein 4 (BRD4), targeting cancer metabolism and BRD4 inhibitors emerge as a promising strategy to prevent tumor relapse.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


