水界面加速协同光- fenton /光催化反应
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
光催化和光-芬顿氧化是很有前途的先进水处理氧化技术。然而,它们相对较慢的动力学速度在很大程度上限制了它们的实际应用。在此,我们在水微滴中进行了协同光催化和光-芬顿反应,以降解有机染料。基于微滴的光反应效率显著提高,在微滴中的降解率为 98.96%,而在大溶液中仅为 38.14%。降解效率的提高得益于微滴中光催化和光-芬顿反应的协同效应。首先,染料(罗丹明 B)和催化剂(g-C3N4 纳米片)在水界面的富集扩大了局部表面浓度,起到了加速反应的作用。其次,水界面自发产生的过氧化氢(17.13 μM)触发了光-芬顿循环,从而在很大程度上促进了 g-C3N4 的电荷分离以及光生电子和空穴的有效利用,从而显著提高了有机染料的降解效率。此外,我们还对单个微滴的反应动力学进行了实时量化。10 μm 微滴中的反应常数为 4.86 × 10-3 s-1,比体相中的反应常数(0.22 × 10-3 s-1)高出 22 倍。这项研究让人们更好地了解了水界面加速光反应,并为解决有机染料降解效率低的问题提供了一种策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
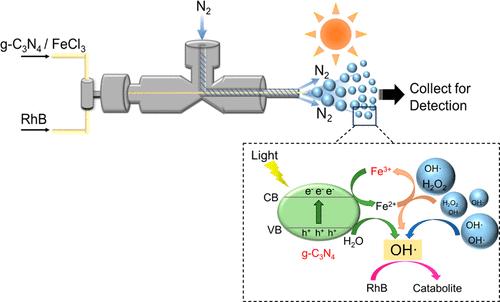
Accelerated Synergistic Photo-Fenton/Photocatalysis Reactions at Aqueous Interfaces
Photocatalysis and photo-Fenton oxidation are promising advanced oxidation technologies for water treatment. Nevertheless, their relatively slow kinetics largely limited their practical applications. Herein, we performed synergistic photocatalysis and photo-Fenton reactions in water microdroplets for the degradation of organic dyes. The efficiency of the microdroplet-based photoreactions was significantly improved with a degradation rate of 98.96% in microdroplets, while it was only 38.14% in the bulk solution. The enhanced degradation efficiency was due to the synergistic effect of the photocatalysis and photo-Fenton reactions in the microdroplets. First, the enrichment of both the dye (rhodamine B) and the catalyst (g-C3N4 nanosheets) at the aqueous interfaces enlarged the local surface concentration, playing a role in the reaction acceleration. Second, the spontaneously generated hydrogen peroxide (17.13 μM) at the aqueous interfaces triggered the photo-Fenton cycle and thus largely promoted the charge separation of g-C3N4 as well as the effective utilization of the photogenerated electrons and holes, leading to a significantly improved degradation efficiency of organic dyes. Further, we quantified the reaction kinetics of individual microdroplets in a real-time manner. The reaction constant in 10 μm microdroplets was 4.86 × 10–3 s–1, which was 22 times higher than that in the bulk phase (0.22 × 10–3 s–1). This study provided a better understanding of accelerated photoreactions at aqueous interfaces and a strategy for addressing the low efficiency of organic dye degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


