靶向蛋白水解嵌合体-阿霉素偶联纳米组件双重治疗EGFR-TKI敏感和耐药非小细胞肺癌。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
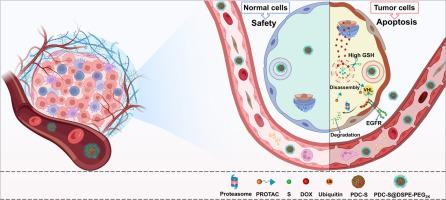
靶向蛋白水解嵌合体(Proteolysis-targeting chimeras, PROTACs)已成为一种有前途的靶向蛋白质降解和药物发现策略。然而,传统的PROTACs面临固有的局限性,也可能有助于诱导耐药性。这些挑战推动了创新战略的发展,以克服这些障碍。为了提高PROTAC-DOX偶联物(PROTAC-DOX conjugates, PDCs)的肿瘤靶向能力,克服了传统protac的缺陷,提出了一种PROTAC-DOX偶联物(PROTAC-DOX conjugates, PDCs)纳米组装策略。所设计的PDC-S纳米颗粒(PDC-S NPs)对耐药菌株表现出强大的抗肿瘤活性(IC50 = 4.7µM),对药敏菌株的体内疗效(TGI = 76%)得到改善,同时副作用最小。此外,PDC-S NPs在肿瘤免疫治疗中具有很大的潜力。本研究为protac -药物偶联物(PDCs)的开发提供了一种新的、有前景的策略。研究意义:我们开发了一种PROTAC-DOX缀合物(PDC)纳米组装策略,以解决传统PROTACs的局限性,如溶解度差、靶向特异性低和耐药。通过自组装构建了PDC-S NPs,这简化了制备过程,并将通常与载体辅助递送系统相关的毒性降至最低。在HCC827细胞中,PDC-S NPs表现出更好的水溶性和细胞摄取,导致EGFR的有效降解。在体内,PDC-S NPs通过EPR效应在肿瘤部位积累,从而增强抗肿瘤效力,减少副作用。此外,PDC-S NPs诱导免疫原性细胞死亡(ICD),抑制PD-L1和VEGF的表达,在肿瘤免疫治疗中具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Proteolysis-targeting chimera-doxorubicin conjugate nanoassemblies for dual treatment of EGFR-TKI sensitive and resistant non-small cell lung cancer
Proteolysis-targeting chimeras (PROTACs) have emerged as a promising strategy for targeted protein degradation and drug discovery. However, traditional PROTACs face inherent limitations and may also contribute to induce drug resistance. These challenges have driven the development of innovative strategies to overcome these obstacles. In current study, a PROTAC-DOX conjugates (PDCs) nanoassembly strategy was introduced to enhance tumor-targeting capability and overcome the drawbacks of conventional PROTACs. The designed PDC-S nanoparticles (PDC-S NPs) demonstrated potent anti-tumor activity against drug-resistant strains (IC50 = 4.7 µM) and improved in vivo efficacy (TGI = 76 %) against drug-sensitive strains, while minimizing side effects. Additionally, PDC-S NPs have great potential in tumor immunotherapy. This study provides a novel and promising strategy for the development of PROTAC-Drug Conjugates (PDCs).
Statement of significance
We developed a PROTAC-DOX conjugates (PDCs) nanoassembly strategy to address the limitations of traditional PROTACs, such as poor solubility, low targeting specificity, and drug resistance. PDC-S NPs were constructed via self-assembly, which simplified preparation and minimized the toxicity typically associated with carrier-assisted delivery systems. The PDC-S NPs showed improved aqueous solubility and cellular uptake, resulting in efficient EGFR degradation in HCC827 cells. In vivo, PDC-S NPs accumulated at tumor sites via the EPR effect, resulting in enhanced anti-tumor potency with reduced side effects. Furthermore, PDC-S NPs induced immunogenic cell death (ICD) and suppressed PD-L1 and VEGF expression, highlighting great potential in tumor immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


