将废活性炭转化为高附加值产品MnO2/C纳米复合材料、氧反应双功能电催化剂和超级电容器电极材料
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
活性炭(AC)用于净化水和空气、除臭、脱色、去污、污水处理、作为储能材料和催化剂等。在使用过程中,活性炭会因孔隙被外来介质堵塞而失去活性,从而产生废活性炭(SAC)。对废活性炭的处理主要有三种方式:再生、用作窑炉燃料和填埋处理。然而,所有这些过程都会对环境造成严重危害。对废活性炭进行再生并将其再次用于类似用途是一件非常昂贵的事情。在这项工作中,通过与不同浓度的 KMnO4 进行水热反应,将滤水器中的 SAC 转化为 MnO2/C 纳米复合材料,并将其用作氧还原和氧进化反应(ORR 和 OER)的电催化剂以及超级电容器的电极材料。XRD、振动和 X 射线光电子能谱研究证实了 SAC 向 MnO2/C 纳米复合材料的转化。使用 KMnO4:SAC 比例为 2:1 合成的 MnO2/C 纳米复合材料具有良好的 ORR(起始电位:0.846 V,电流密度:-7.59 mA cm-2)和 OER(过电位为 445 mV,电流密度为 10 mA cm-2)活性。此外,它还显示出良好的电容性能(1 A g-1 时为 252 F g-1,速率保持率和稳定的循环寿命超过 10,000 次)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
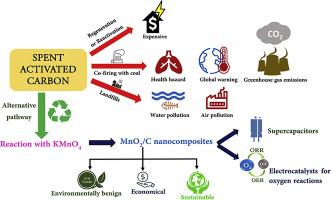
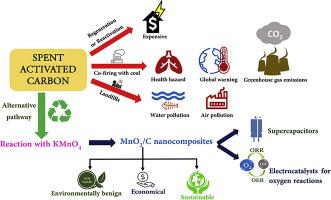
Conversion of spent activated carbon into a value-added product, MnO2/C nanocomposite, a bi-functional electrocatalyst for oxygen reaction and an electrode material for supercapacitors
Activated carbon (AC) is used for purification of water and air, deodorization, decolouration, decontamination, sewage treatment, as energy storage materials, catalysts, etc. AC loses its activity during usage due to the clogging up of pores by foreign media, resulting in spent activated carbon (SAC). SAC is primarily treated in three ways: regeneration, use as fuel in kilns and landfill disposal. However, all these processes cause severe harm to the environment. Regenerating SAC and using it again for a similar purpose is an expensive affair. In this work, the SAC from a water filter is converted into MnO2/C nanocomposites by hydrothermal reaction with KMnO4 of different concentrations and used as electrocatalysts for oxygen reduction and oxygen evolution reactions (ORR and OER) and as an electrode material in supercapacitors. The conversion of SAC to MnO2/C nanocomposites is confirmed by XRD, vibrational and X-ray photoelectron spectroscopic studies. MnO2/C nanocomposite synthesized using KMnO4:SAC ratio of 2:1 demonstrates a good ORR (onset potential: 0.846 V and current density:7.59 mA cm-2) and OER (overpotential of 445 mV to reach 10 mA cm-2) activities. Also, it displays good capacitive performance (252 F g-1 at 1 A g-1, rate retention and stable cycle life over 10,000 cycles).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


